When the test pilot of the Airbus A380 lifted off for the first time from the runway, the sole “in vitro” test in a wind tunnel that had been performed, was on a small miniaturised model (scale 1/39). Prior to that, the whole airplane had been designed and tested “in silico” with the use of computational fluid dynamics, which is a major tool for aerodynamic shape design (Figure 1).
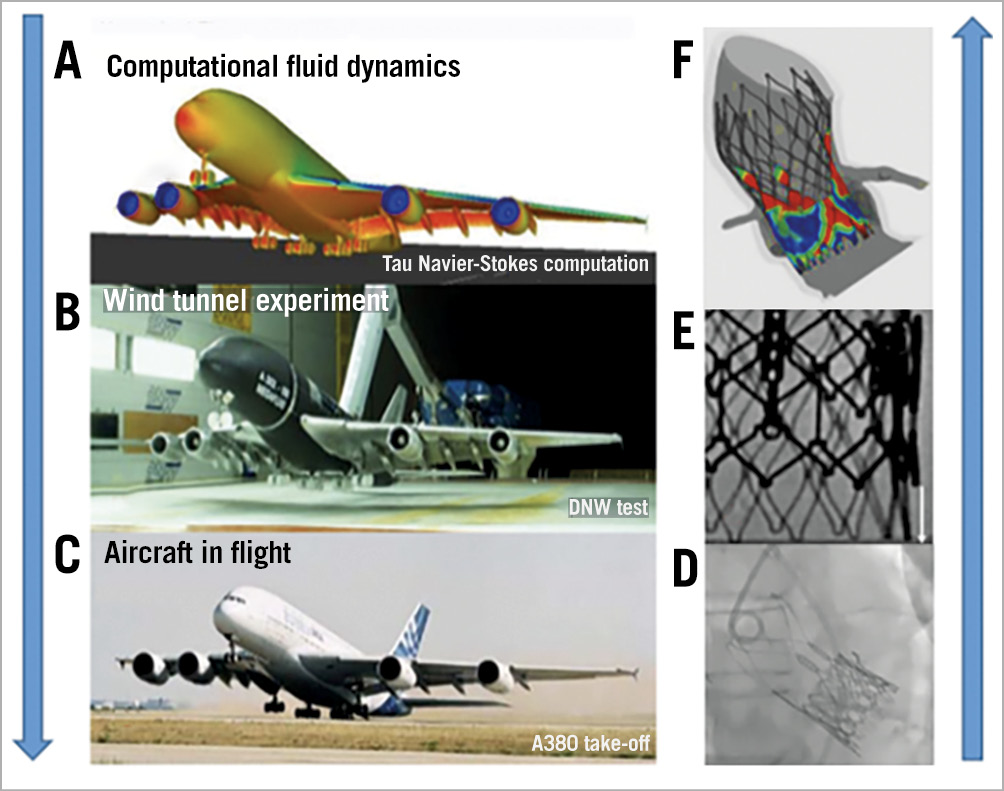
Figure 1. Development and testing steps. A) Computational fluid dynamics testing of the Airbus A380. B) Wind tunnel testing of the miniaturised model. C) Real take-off. © AIRBUS S.A.S. D) in vivo THV-in-THV. E) Present in vitro THV-in-THV testing. F) Predicted PVL after THV from patient-specific computational modelling and simulation6. PVL: paravalvular leak; THV: transcatheter heart valve
In the past, we physicians followed the opposite pathway for the development of a new technique and performed the first valve-in-valve (ViV) in vivo in patients before trying to understand the nature of the procedure in vitro.
In February 2005, ViV implantation was performed for the first time as a bailout in a patient to correct major malpositioning1. Subsequently, in 2007, in vivo testing in pigs was conducted to assess the technical feasibility of transcatheter ViV implantation using a Cribier-Edwards transcatheter heart valve (THV) (Edwards Lifesciences, Irvine, CA, USA) in a Carpentier-Edwards surgical heart valve (SHV) (Edwards Lifesciences) in the aortic and mitral positions2. In the same year, the first clinical ViV (transcatheter aortic valve replacement [TAVR] in surgical aortic valve replacement [SAVR]) case was performed using a Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) in a Mitroflow bioprosthesis (Sorin Group Inc., Saluggia, Italy)3.
ViV TAVR has emerged as an acceptable option for reintervention in patients with malfunctioning bioprosthetic valves (THV or SHV).
In the first place, operators have to be reminded of the relationship of de novo implanted valve leaflets to the aortic valve annulus in a balloon-expandable (BEV) and self-expanding (SEV) THV; the leaflets in a BEV are located at intra-annular level, whereas the leaflets of contemporary SEV are located at either a supra- or intra-annular level. At the time of repeat TAVR it is essential to ensure the apposition of pre-existing leaflets against the metallic platform to avoid leaflet overhang while maintaining coronary access (Figure 2).
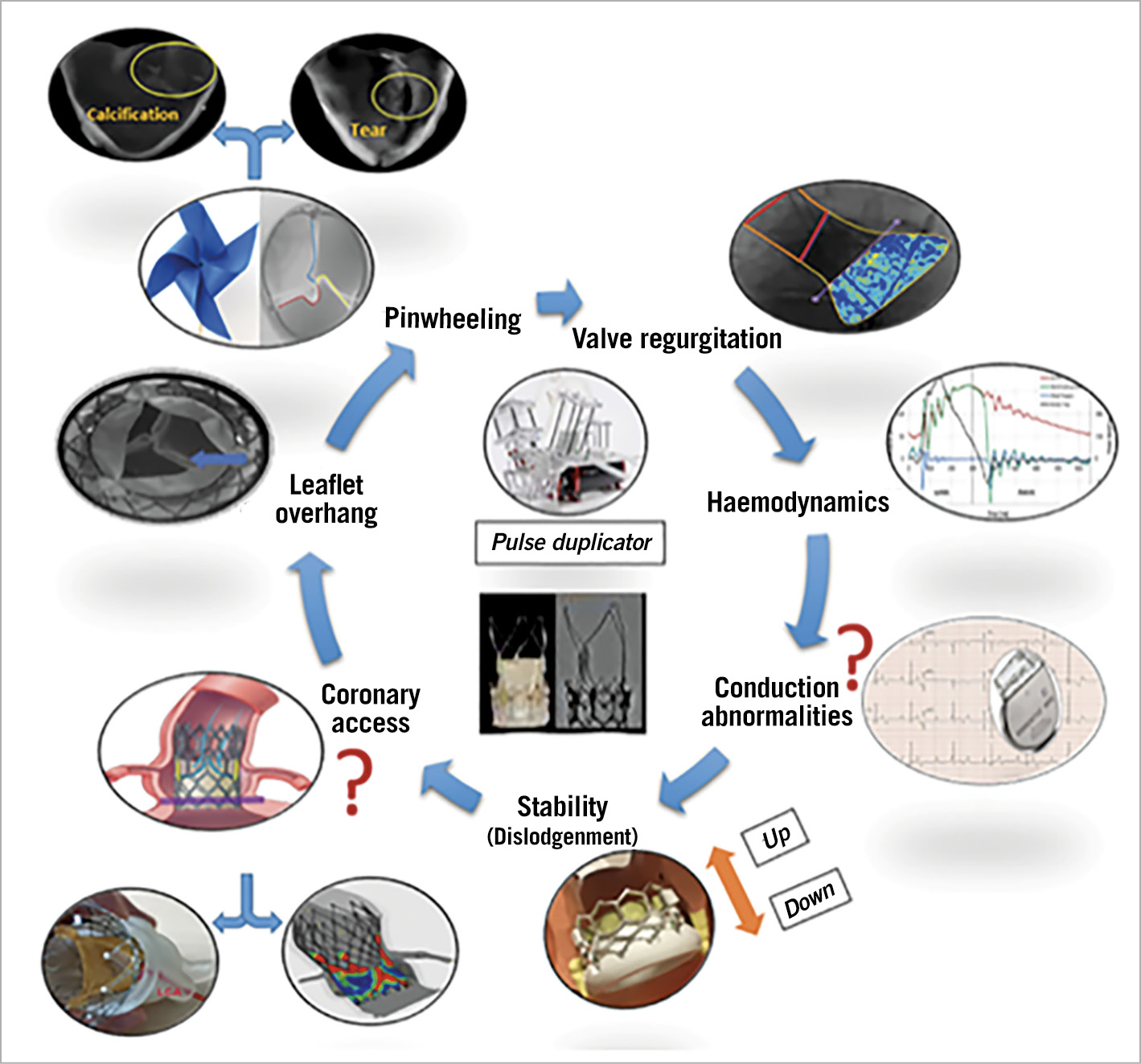
Figure 2. The various parameters that need to be tested for the assessment of ViV performance.
In this issue of EuroIntervention, Sathananthan et al4 present a comprehensive and detailed in vitro analysis of repeat TAVR with a variety of THV-in-THV.
The pressure gradient (PG), paravalvular leakage (PVL) and effective orifice area (EOA) were measured in a Pulse Duplicator (ViVitro Labs, Inc.,Victoria, BC, Canada); the ViV positioning was documented by high-resolution photography and fluoroscopy, while the pinwheeling was dynamically imaged with a high-speed camera.
Conventional haemodynamic parameters (PG, PVL and EOA) were acceptable overall, but other specific issues such as device dislodgement, pinwheeling and overhanging leaflets were encountered. These three issues can be overcome by precise positioning and optimal expansion.
The pinwheeling effect incriminated in structural valve deterioration is a most subtle alteration of the leaflets which can only be documented in an in vitro setting by high-speed camera and escapes in vivo clinical imaging; therefore, the experimental findings of the authors and their ensuing recommendations have to be considered seriously.
In their paper, the authors reported four different major strategies with various valve designs and sizes: BEV-in-BEV, SEV-in-BEV, BEV-in-SEV and SEV-in-SEV.
Overall a high implantation (+4 mm from the lower THV edge) of SEV in a BEV is recommended.
The large ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) had a high regurgitant fraction (RF%) when implanted in a SAPIEN XT (Edwards Lifesciences) at 0 mm and –4 mm depth.
Of note, the ALLEGRA™ THV (New Valve Technology, Hechingen, Germany) exhibits similar pinwheeling irrespective of implantation depth.
No notable pinwheeling was observed when a SAPIEN 3 (Edwards Lifesciences) or Evolut™ PRO (Medtronic) were implanted in an Evolut™ R (Medtronic).
Implantation of a BEV-in-BEV results in severe pinwheeling.
One of the unavoidable weaknesses of this experimental setting is the absence of surrounding anatomic structure (aortic root and coronaries), so the impact of the various tested strategies on coronary access and conduction disturbance remain unpredictable since they cannot be tested in vitro.
Recently, Percy et al reported 0.46% incidence of repeat TAVR in a national registry between 2012 and 2017. Hospital stay, major bleeding, acute kidney injury and the 30-day major adverse cardiac events (MACE) rate including mortality were significantly lower with repeat TAVR compared with SAVR after THV surgical explantation, although the one-year mortality was similar5.
There is still a multitude of implantation options that need to be tested, but can’t be obtained solely from in vitro testing, and will need more sophisticated and comprehensive testing methods6.
In the near future, we will entirely simulate the whole procedure in silico with computational modelling and simulation in order to find out the optimal device type, size and positioning, ultimately to make the clinical outcome more predictable.
The sky is the limit. Let us plan for future take-offs… (Figure 1).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

