Abstract
Background: THV implantation within failed surgical valves is well established. However, the implications of THV implantation within failed THVs are poorly understood.
Aims: This study aimed to assess the impact of different transcatheter heart valve (THV) designs and implant positioning strategies on hydrodynamic performance after redo transcatheter aortic valve implantation (TAVI).
Methods: THVs of varying design (SAPIEN 3, Evolut PRO, ACURATE neo, ALLEGRA, and Portico) and size were implanted inside SAPIEN XT and Evolut R THVs. Hydrodynamic function as per International Organization for Standardization (ISO) specifications was evaluated using a pulse duplicator, and multi-modality imaging was performed.
Results: The majority of tested THV-in-THV combinations resulted in stable anchoring of the new implant. However, some combinations resulted in unstable anchoring with the potential for dislodgement or embolisation. Hydrodynamic function was favourable for all tested THV designs implanted in the intra-annular SAPIEN XT THV. SAPIEN 3 implantation within an Evolut THV with supra-annular leaflets must be appropriately sized to ensure adequate anchoring. Avoidance of an intra-annular deployment mitigated leaflet overhang of the Evolut leaflets and optimised hydrodynamic performance.
Conclusions: This study demonstrates that most THV designs and implantation strategies can result in favourable hydrodynamic performance following redo TAVI. Whether the leaflets of a failed THV are intra- or supra-annular may have important implications for the positioning of a redo-THV implant. Certain THV designs or implantation positions may be more desirable when performing redo TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) has been shown to be an effective therapy for patients with severe symptomatic aortic sten-osis irrespective of surgical risk1,2,3,4,5,6. As TAVI is increasingly utilised in lower-risk patients with the potential for longevity, trans-catheter heart valve (THV) durability and the possibility of TAVI repeatability become increasingly relevant. Although THV implantation within failed surgical valves is well established7, the implications of THV implantation within failed THVs are poorly understood.
We evaluated the implications for valve performance after redo TAVI due to variations in THV design, sizing, and positioning.
Methods
The study was performed at the Centre for Heart Valve Innovation Bench Testing Laboratory (St Paul’s Hospital, Vancouver, Canada) and at ViVitro Labs Inc (Victoria, Canada).
VALVES
The potential for redo TAVI in failed SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) and Evolut™ R (Medtronic, Minneapolis, MN, USA) THVs was evaluated. These two valves were deployed as specified by manufacturer recommendations. Five types of THV for repeat TAVI were studied: SAPIEN 3 (S3) (Edwards Lifesciences), CoreValve® Evolut™ PRO (Medtronic), ACURATE neo™ (ACn; Boston Scientific, Marlborough, MA, USA), Portico™ (Abbott Vascular, Santa Clara, CA, USA) and ALLEGRA (New Valve Technology, Hechingen, Germany).
The design characteristics of the SAPIEN XT, S3, Evolut R, Evolut PRO, ACn, Portico and ALLEGRA have been described previously8,9,10,11,12,13.
EX VIVO REDO TAVI IN SAPIEN XT
The full range of sizes of SAPIEN XT valves was utilised. Into these were implanted S3, Evolut PRO, ACn, ALLEGRA and Portico THVs. For implantation of an S3 into a SAPIEN XT, the S3 was positioned with the outflow approximately 20% above the outflow of the SAPIEN XT to maximise the effective orifice area and reduce the residual transvalvular gradient14. The top of the SAPIEN XT frame was used as the reference for the outflow. Implantation of a self-expanding THV (Evolut, ACn, ALLEGRA, Portico) was assessed at three different implantation depths (+4 mm, 0 mm and –4 mm) using the inflow of the SAPIEN XT as a reference (Figure 1).
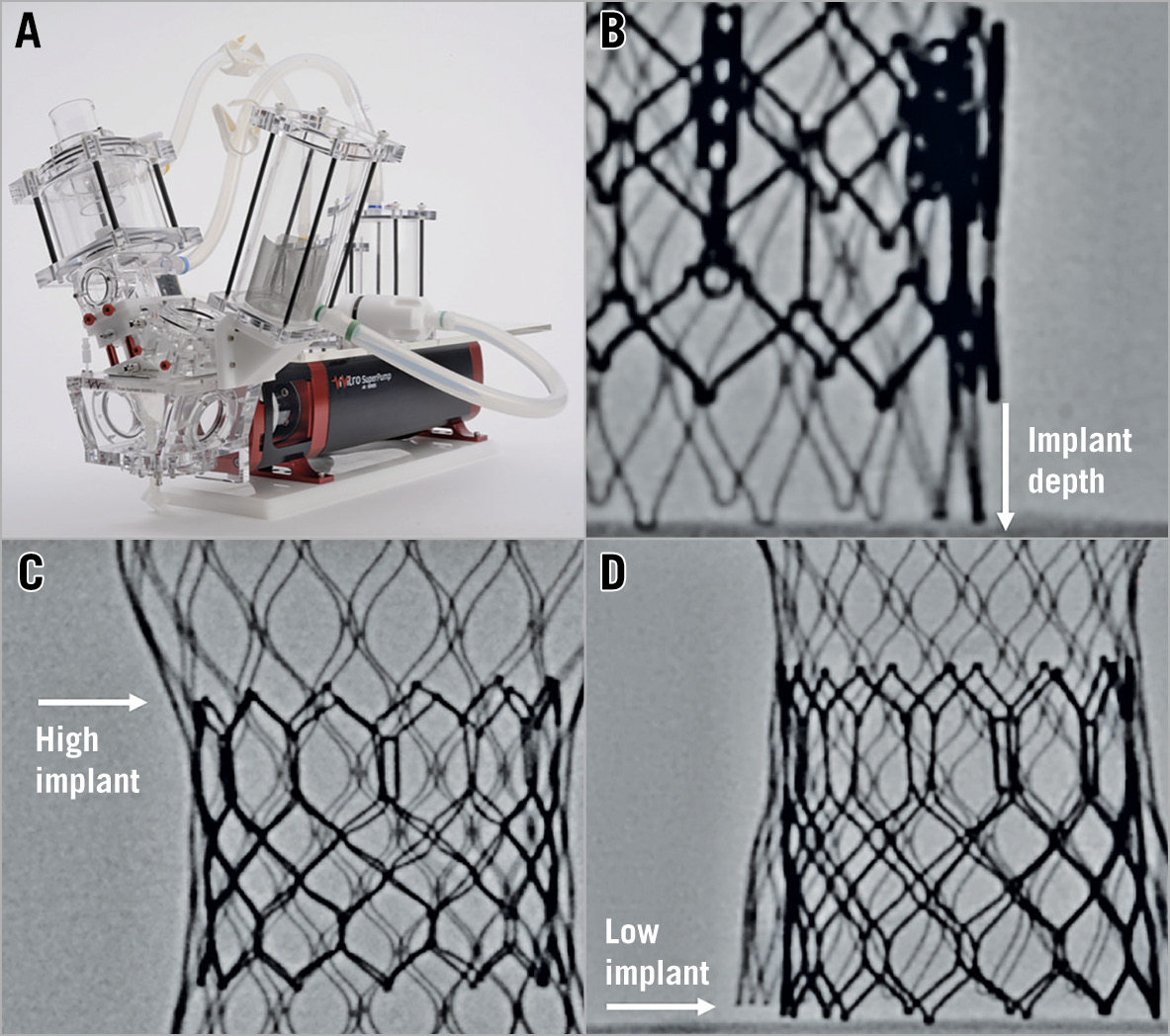
Figure 1. Bench testing methodology. A) Pulse duplicator used for hydrodynamic testing. B) Implantation depth of the new THV was measured from the inflow of the SAPIEN XT/Evolut R. C) For a high implantation of an S3 into an Evolut R, the top of the S3 was positioned just above the level of the outflow of the Evolut R leaflets. D) For a low implantation of an S3 into an Evolut R, the S3 was positioned relative to the inflow of the Evolut R frame.
A 23 mm S3, 26 mm Evolut, small ACn, 23 mm ALLEGRA and 25 mm Portico were implanted into a 23 mm SAPIEN XT. A 26 mm S3, 29 mm Evolut, medium ACn, 27 mm ALLEGRA, and 29 mm Portico were implanted into a 26 mm SAPIEN XT. A 29 mm S3, large ACn, 27 mm ALLEGRA, and 29 mm Portico valve were implanted into a 29 mm SAPIEN XT. A 34 mm Evolut and 31 mm ALLEGRA were not available for this bench study (Figure 2).
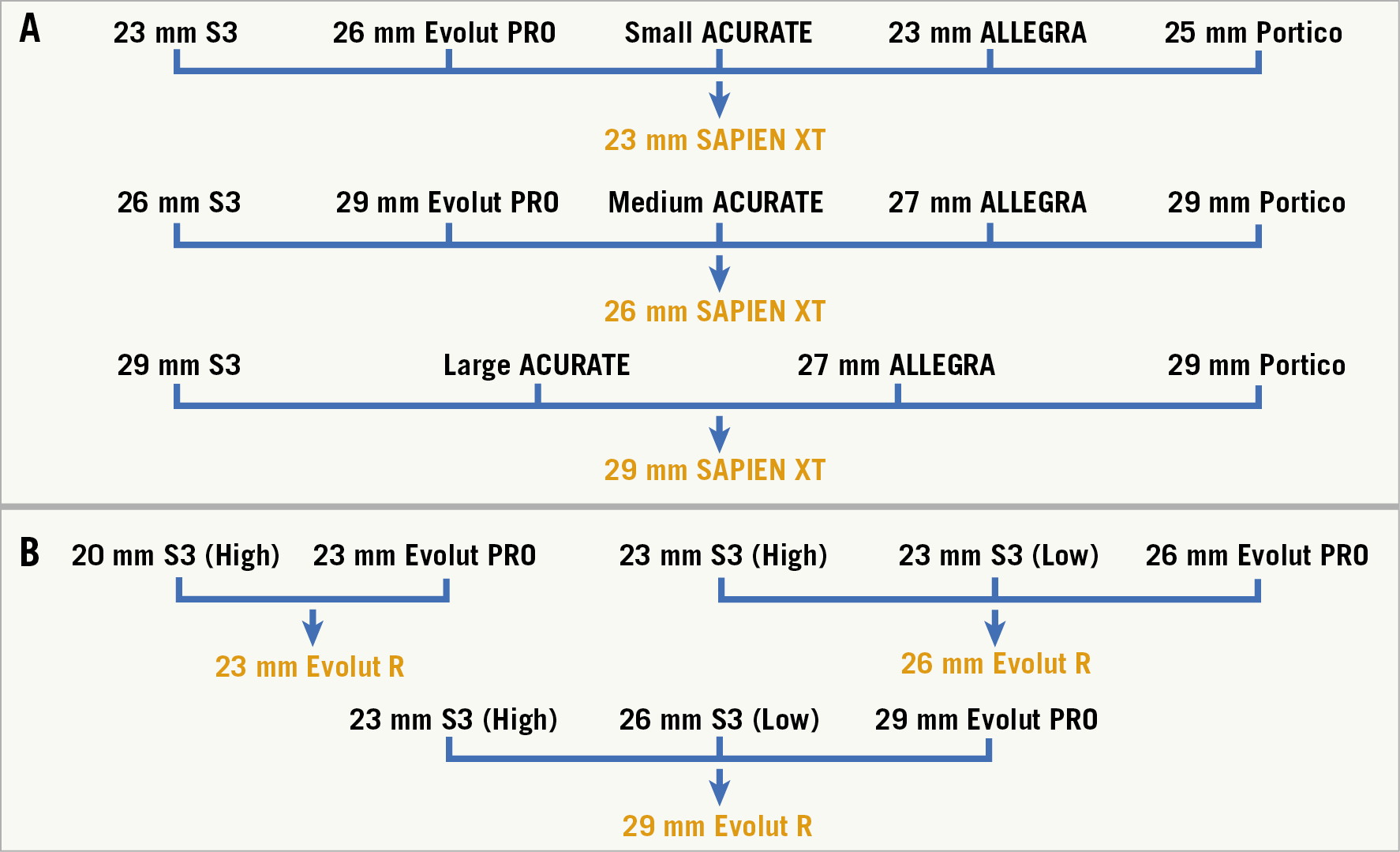
Figure 2. Ex vivo redo TAVI in a SAPIEN XT and Evolut R. A) Ex vivo redo TAVI in SAPIEN XT. Implantation of a self-expanding THV (Evolut, ACn, ALLEGRA, Portico) was assessed at three different implantation depths (+4 mm, 0 mm and –4 mm) using the inflow of the SAPIEN XT as a reference. B) The Evolut R model utilised 23 mm, 26 mm and 29 mm THVs. Into these were implanted S3 and Evolut PRO THVs. Implantation of an Evolut PRO into an Evolut R was assessed at three different implantation depths (+4 mm, 0 mm and –4 mm) using the inflow of the Evolut R as a reference.
EX VIVO REDO TAVI IN EVOLUT
The Evolut R model utilised 23 mm, 26 mm and 29 mm THVs. Into these were implanted S3 and Evolut PRO THVs. Implantation of an Evolut PRO into an Evolut R was assessed at three different implantation depths (+4 mm, 0 mm and –4 mm) using the inflow of the Evolut R as a reference. Implantation of an S3 into an Evolut R was assessed in two different implantation positions, high and low. For a high implantation, the S3 was positioned at the level of the outflow of the Evolut R leaflets. For a low implantation, the S3 was positioned relative to the inflow of the Evolut R frame.
A 20 mm S3 was implanted high in a 23 mm Evolut. Ideally a 20 mm S3 should be implanted in a low position in a 23 mm Evolut, but an additional 20 mm S3 valve was unavailable for the bench test. A 23 mm S3 was implanted both high and low into an Evolut. A 23 mm and a 26 mm S3 were implanted in a high and low position, respectively, for a 29 mm Evolut (Figure 1). The choice of S3 sizing for redo TAVI into an Evolut PRO was based on the dimensions of the Evolut frame. The waist of a 23 mm, 26 mm and 29 mm Evolut PRO are 20 mm, 22 mm and 23 mm, respectively.
In the case of the S3 THV, nominal volume was utilised in the delivery system as specified by the manufacturer's recommendations. If there were any cases of valve dislodgement or embolisation, the effect of overexpansion was assessed. Ideally, if valve dislodgement was observed for deployment strategies of an S3 into an Evolut, the same strategy would have been re-tested with use of a larger sized S3 to assess anchoring and function. However, given the difficulty of acquiring THVs for the purposes of bench testing, we overfilled the delivery system as a surrogate to assess the impact of implantation of a larger S3 on anchoring. The S3 valves were therefore initially redeployed with an additional 1 ml of volume in the 23 mm S3, aiming to achieve overfilling by approximately 10%15. Incremental volume would be added if anchoring was still insufficient. The nominal inflation volume for a 23 mm S3 is 17 ml.
IMAGING
High-resolution photography was performed at the same magnification and same fixed camera height. Fluoroscopy was performed using a standard adult cardiac catheterisation laboratory (General Electric Healthcare, Chicago, IL, USA).
HYDRODYNAMIC ASSESSMENT
Hydrodynamic testing was performed for each sample, using a commercially available pulse duplicator (ViVitro Labs Inc, Victoria, Canada) (Figure 1). Valves were tested in accordance with International Organization for Standardization (ISO) 5840-3:2013 guidelines regarding in vitro pulsatile flow testing for heart valve substitutes implanted by transcatheter techniques16. Valves were placed in a holder fabricated from silicone with a durometer of scale Shore A hardness of 40±5. Justification for the selection of sample holder hardness was based on published data on acceptable tissue compliance17,18,19. The test fluid used was 0.9±0.2% sodium chloride test solution maintained at 37±2°C (one drop of Cosmocil® [preservative] per 1 L).
Valves were tested on the aortic side of the pulse duplicator with a spring-loaded disc valve (ViVitro Labs) on the mitral side of the pulse duplicator. Measurements were based on average results taken from 10 consecutive cycles. High-speed video was captured at each step condition. Pulsatile forward flow performance was tested at a nominal beat rate of 70±1 beats per minute, systolic duration of 35±5%, mean aortic pressure of 100±2 mmHg, and simulated cardiac outputs of 5±0.1 litres per minute. Mean gradient (MG) (mmHg), regurgitant fraction (RF) (%) and effective orifice area (EOA) (cm2) were assessed. Valve dislodgement or embolisation was noted during testing of redo TAVI samples.
The minimum ISO standards for EOA for a 23 mm, 25 mm, 27 mm, and 29 mm deployed diameter within the implant site are 1.25 cm2, 1.45 cm2, 1.7 cm2 and 1.95 cm², respectively. The minimum ISO standard for total RF (including paravalvular leakage) is <20%16.
PINWHEELING
Pinwheeling, as defined by the ISO guideline for THV testing, refers to twisting of the leaflet-free edges resulting from excessive leaflet redundancy16. The degree of pinwheeling was based on the high-speed videos with backward pressure.
ETHICS
This study was a purely bench study with no human or animal participants; ethics approval was not required.
STATISTICAL ANALYSIS
Hydrodynamic variables are reported as mean±standard deviation (SD).
Results
The majority of tested THV designs, sizes and positioning had stable anchoring during hydrodynamic testing. Of note, the 27 mm ALLEGRA had insufficient anchoring and the 29 mm Portico THVs embolised at all tested implantation depths in a 29 mm SAPIEN XT. When a small S3 was implanted into an Evolut there was insufficient anchoring. A 20 mm S3 in a 23 mm Evolut R, and a 23 mm S3 in a 26/29 mm Evolut R at nominal volume resulted in dislodgement when implanted in a high position (Figure 3). When the S3 was implanted high in an Evolut R with additional volume in the delivery system to simulate a larger THV, dislodgement or embolisation was not observed.
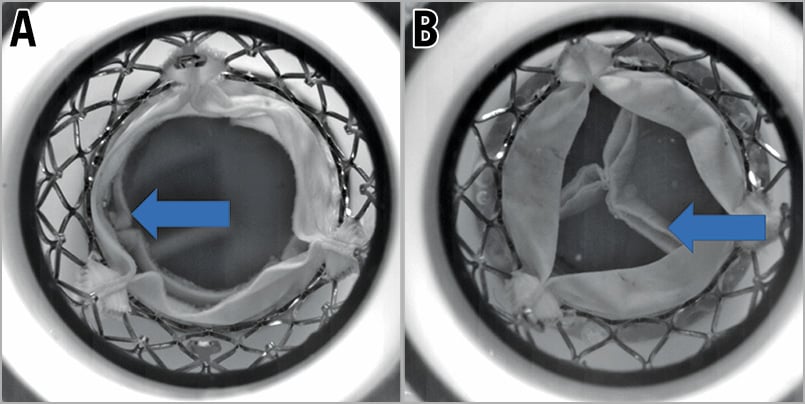
Figure 3. Dislodgement of S3 samples when implanted high within an Evolut R at a nominal volume. A) Dislodgement of a 20 mm S3 positioned high inside a 23 mm Evolut R. The arrow indicates the leaflets of the S3 which are now below the level of the Evolut R leaflet due to dislodgement of the S3 in a ventricular direction. B) Dislodgement of a 23 mm S3 positioned high inside a 26 mm Evolut R. The arrow indicates the leaflets of the S3 which are now below the level of the Evolut R leaflet due to dislodgement of the S3 in a ventricular direction.
TRANSVALVULAR GRADIENT
All THV designs and implantation depths had acceptable transvalvular gradients for samples implanted into a 23 mm, 26 mm and 29 mm SAPIEN XT (Figure 4-Figure 6). The ACn had a lower gradient when implanted high (+4 mm). Implantation depth did not have an impact on transvalvular gradient for the Evolut PRO, ALLEGRA and Portico. Transvalvular gradients were also favourable for implantation of an S3 and Evolut PRO into an Evolut R, irrespective of implantation depth (Figure 7).
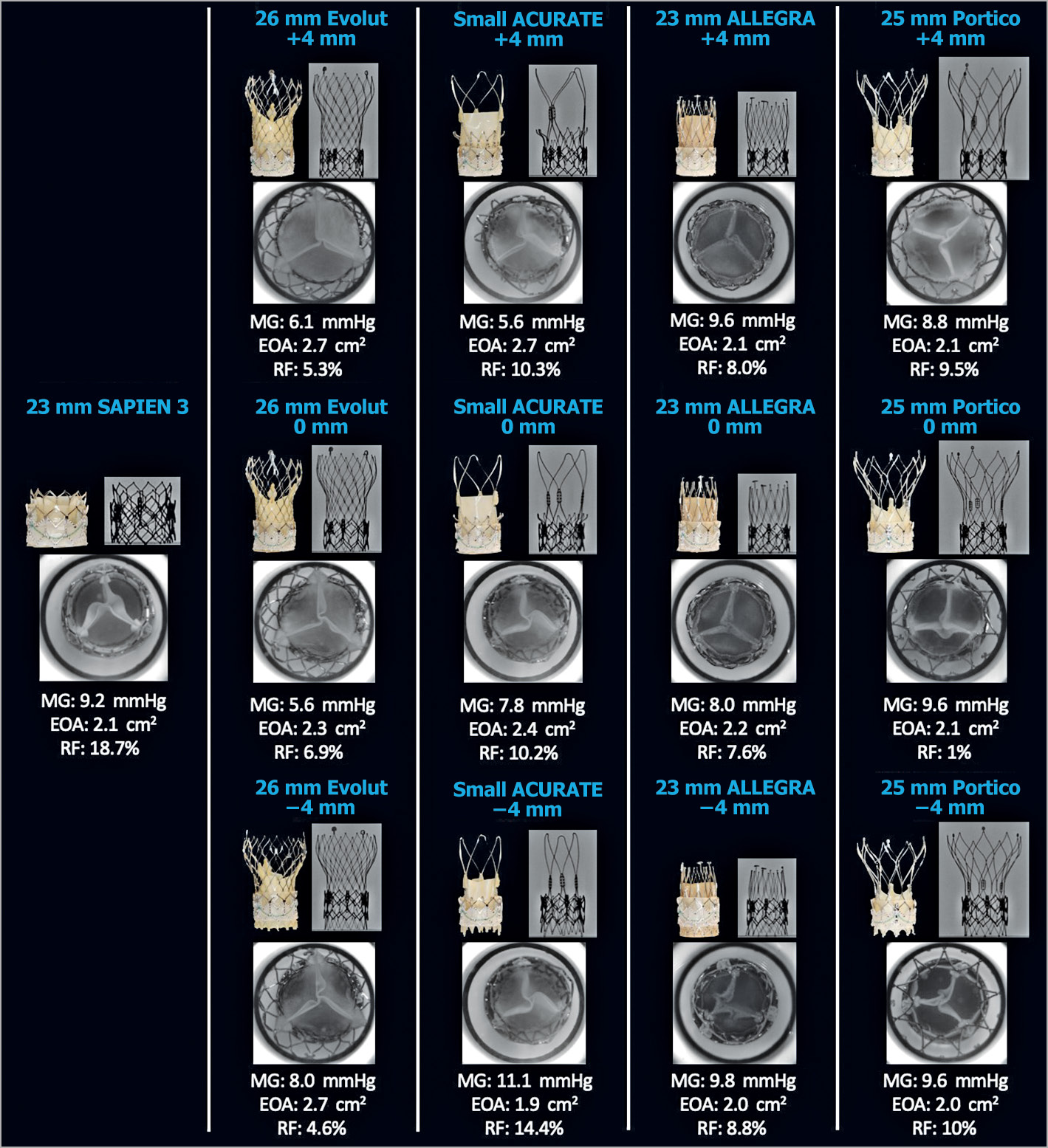
Figure 4. Hydrodynamic performance and multimodality imaging of different THVs in a 23 mm SAPIEN XT. A 23 mm S3, 26 mm Evolut, small ACn, 23 mm ALLEGRA, and 25 mm Portico were implanted into a 23 mm XT and hydrodynamic function was assessed. EOA: effective orifice area (cm2); MG: mean gradient; RF: regurgitant fraction
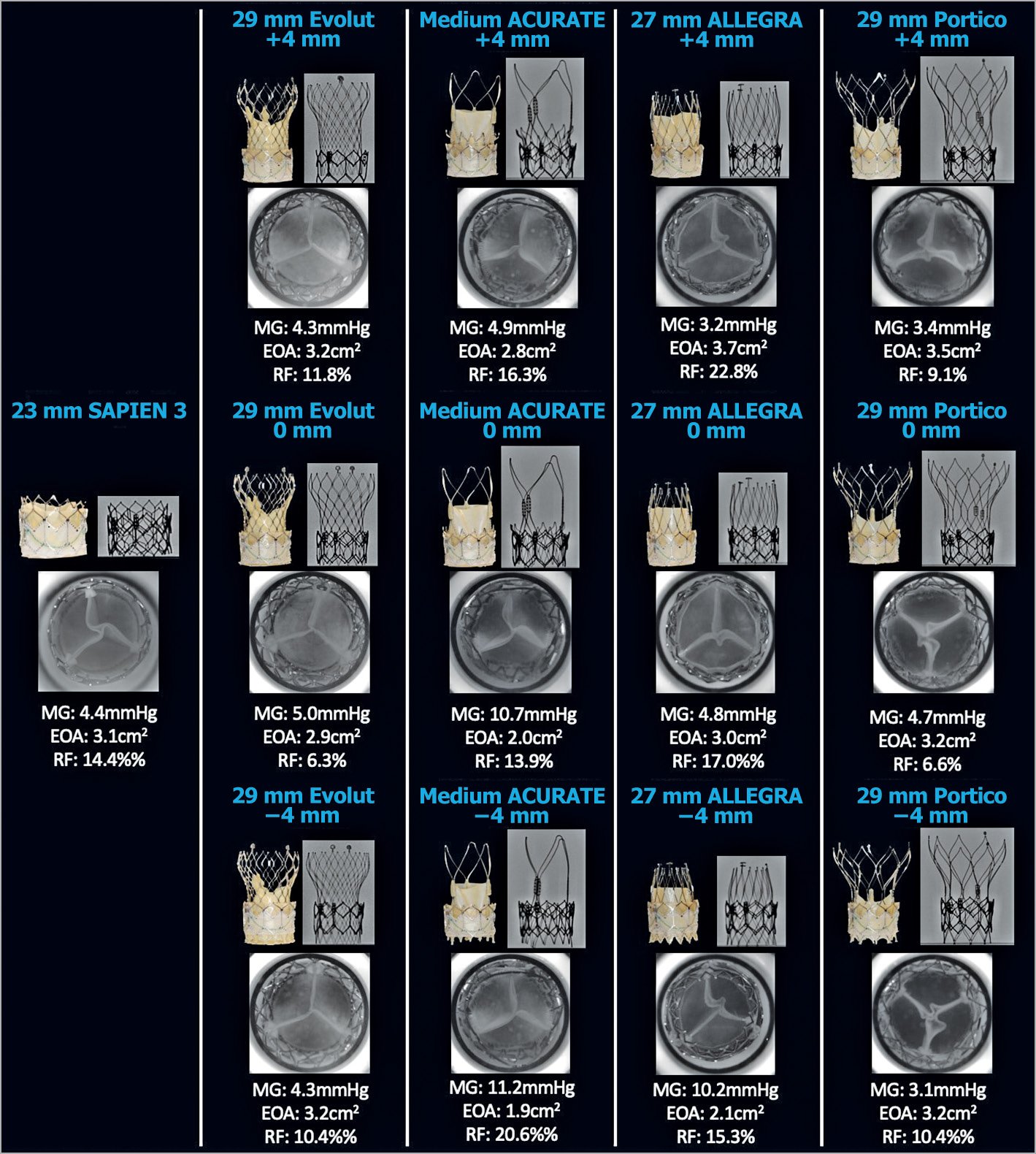
Figure 5. Hydrodynamic performance and multimodality imaging of different THVs in a 26 mm SAPIEN XT. A 26 mm S3, 29 mm Evolut, medium ACn, 27 mm ALLEGRA, and 29 mm Portico were implanted into a 26 mm XT and hydrodynamic function was assessed. EOA: effective orifice area (cm2); MG: mean gradient; RF: regurgitant fraction
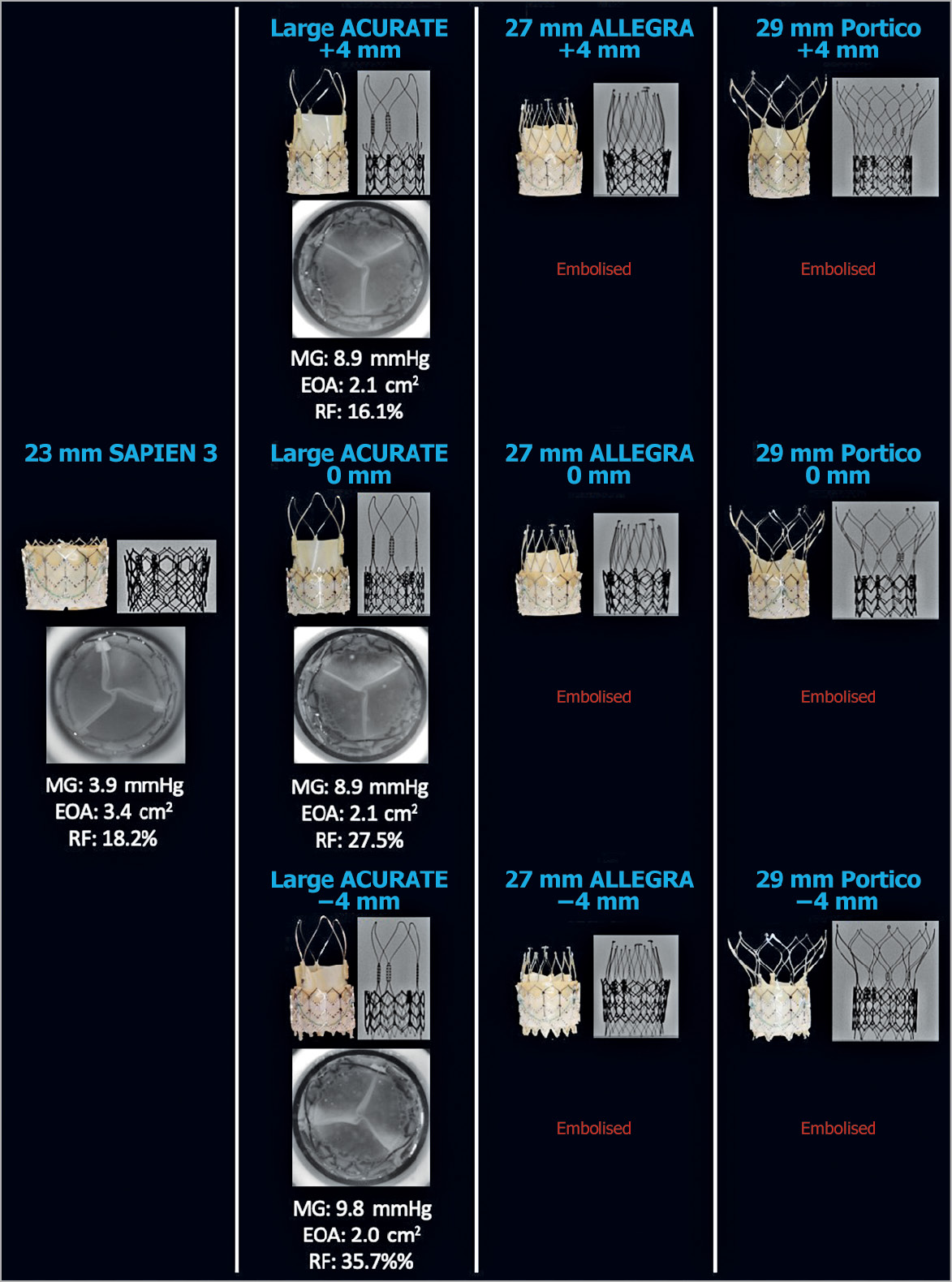
Figure 6. Hydrodynamic performance and multimodality imaging of different THVs in a 29 mm SAPIEN XT. A 29 mm S3, large ACn, 27 mm ALLEGRA, and 29 mm Portico were implanted into a 29 mm XT and hydrodynamic function was assessed. The 27 mm ALLEGRA and 29 mm Portico embolised at all tested implantation depths. EOA: effective orifice area (cm2); MG: mean gradient; RF: regurgitant fraction
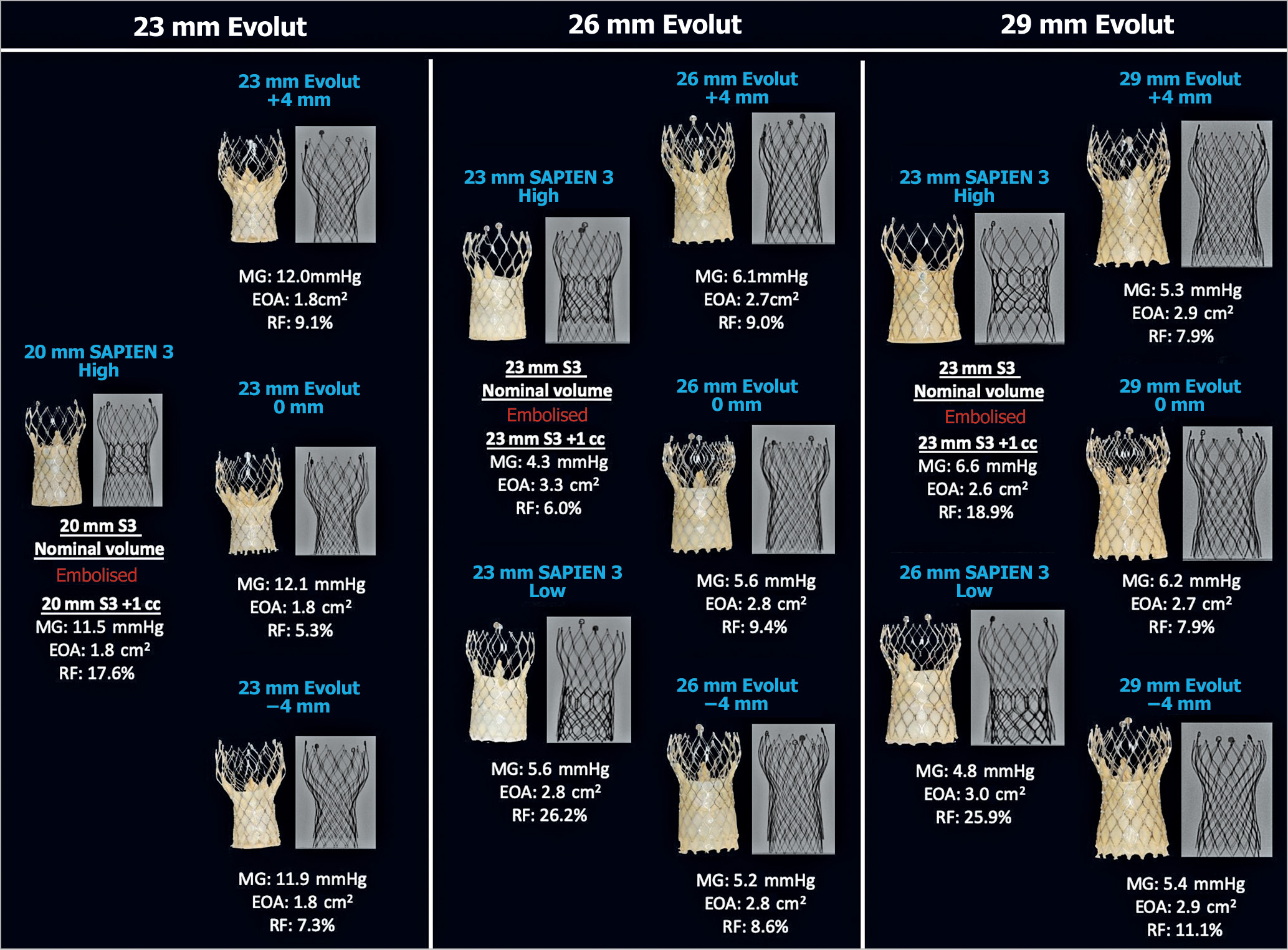
Figure 7. Hydrodynamic performance and multimodality imaging of different THVs into 23 mm, 26 mm and 29 mm Evolut R. A 23/26 mm S3 and 29 mm Evolut were implanted into a 29 mm Evolut. EOA: effective orifice area (cm2); MG: mean gradient; RF: regurgitant fraction
TRANSVALVULAR REGURGITANT FRACTION
All THV designs and implantation depths had an acceptable RF (<20%) when implanted in a 23 mm and 26 mm SAPIEN XT (Figure 4, Figure 5). In the 29 mm SAPIEN XT, the 29 mm S3 and large ACn at +4 mm implant depth had acceptable RF. The large ACn had a high RF when implanted at 0 mm (27.5%) and –4 mm (35.7%) depth.
The RF was high when an S3 was implanted low in a 26 mm (26.2%) and a 29 mm (25.9%) Evolut R (Figure 7). High-speed video demonstrated that a low S3 implantation in an Evolut R resulted in cycle-to-cycle variation during hydrodynamic testing, with evidence of impaired and variable leaflet closure with backward pressure (Figure 8, Moving image 1).
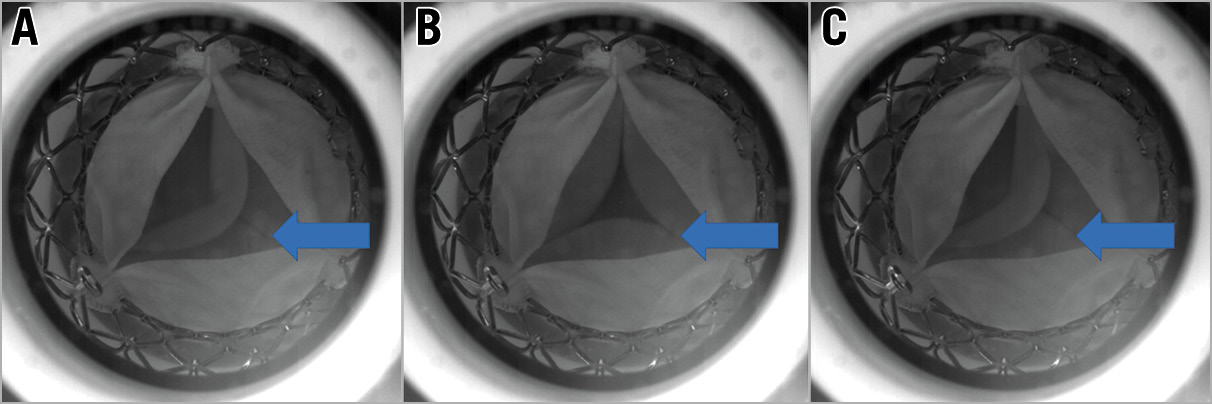
Figure 8. High-speed video images demonstrating variation in leaflet coaptation of an S3 implanted low in an Evolut R. Each panel shows the cycle-to-cycle variation observed following an S3 implanted low in an Evolut R. There is variation in leaflet coaptation of the S3 leaflets with backward pressure. The arrows indicate the S3 leaflets. A) & C) Impaired leaflet coaptation. B) Impaired leaflet coaptation leading to a central coaptation defect with maximal backward pressure.
EFFECTIVE ORIFICE AREA
All THV designs and implantation depths had acceptable EOAs as per ISO standards for samples implanted into a 23 mm, 26 mm and 29 mm SAPIEN XT (Figure 4-Figure 6). The ACn had a higher EOA when implanted high (+4 mm). Implantation depth did not have a notable impact on EOA for the Evolut PRO, ALLEGRA and Portico. EOAs were also favourable for implantation of an S3 and Evolut PRO into an Evolut R, irrespective of implantation depth (Figure 7).
PINWHEELING
At high implantation (+4 mm), the degree of pinwheeling was less with self-expanding THVs (Evolut R, ACn, ALLEGRA and Portico) when implanted in a SAPIEN XT. With lower implantation, there was progressively worse leaflet pinwheeling, observed particularly in the Evolut, ACn and Portico THVs in a SAPIEN XT. The degree of pinwheeling was similar in the ALLEGRA THV irrespective of implantation depth. S3s implanted in a SAPIEN XT all had a degree of pinwheeling, which was severe in the 23 mm and 26 mm SAPIEN XT. There was no notable pinwheeling when an S3 or Evolut was implanted in an Evolut R.
Discussion
As TAVI becomes more common in patients with longer life expectancy, strategies to deal with failed THVs will be of increasing importance. The importance of THV design, sizing and positioning must be understood when performing redo TAVI. This study demonstrates that certain THV designs or implantation positions may be more desirable than others when performing redo TAVI.
A large multicentre series has demonstrated that redo TAVI can be performed safely for selected patients with valve dysfunction after TAVI20. However, it appears that residual aortic regurgitation is more common than that observed after valve-in-valve (VIV) intervention in failed surgical valves7. Our study provides insights that can provide guidance regarding specific THV design and implantation technique that may lead to suboptimal performance. This may aid implanting physicians when performing redo TAVI. In this study, most combinations of redo TAVI had favourable hydrodynamic performance. This is consistent with the large multi-centre clinical experience that has been reported. However, some samples lead to suboptimal performance. Specifically, implantation of an appropriately sized short frame THV into a tall frame valve with supra-annular leaflets is important when performing redo TAVI. A high S3 implantation, while desirable in order to avoid “leaflet overhang” of the failed Evolut R, was associated with valve dislodgement when a small S3 was deployed with nominal volume. Ideally, testing would have been performed with a larger S3; however, acquisition of THVs for independent bench testing is challenging. Therefore, we utilised overexpansion as a surrogate for a larger-sized THV. When the S3 delivery system was overfilled with additional volume, there was no dislodgement or embolisation observed. Therefore, a larger S3 with sizing based on the annulus may lead to better anchoring in a high position. In this study, the S3 sizing for a high implantation was based on the waist dimensions of the Evolut R. Equally of concern is low implantation of an S3 in an Evolut R, which resulted in leaflet overhang of the Evolut R leaflets. A high RF was noted in these samples with evidence of cycle-to-cycle variation in leaflet coaptation. The mechanism of this high RF may be related to leaflet interaction of the S3 leaflet with either the Evolut R frame or leaflet. Similar leaflet interaction has been observed with the ACURATE neo during VIV interventions in failed surgical valves12. Alternatively, there may be a flow phenomenon due to backward flow through the overhanging Evolut R leaflets that subsequently compromises leaflet coaptation of the underlying S3 leaflets.
While some THV designs have similar features such as self-expanding deployment or supra-annularly positioned leaflets, ultimately each design must be assessed on its own merits and one must not assume a class effect based on mechanism of valve deployment. In this study, each self-expanding valve had its own unique performance during redo TAVI in a SAPIEN XT. In general, the Evolut R and ACn had superior performance when positioned high within the SAPIEN XT. In contrast, a low implantation with the ACn led to suboptimal expansion of the upper crown of the ACn, which was constrained within the frame of the SAPIEN XT, and impacted on hydrodynamic performance. This has also been demonstrated previously when the ACn is positioned low during VIV intervention in a failed surgical valve12. A self-expanding valve positioned low in a SAPIEN XT also resulted in pinwheeling, particularly in the smaller 23 mm SAPIEN XT THV. There was also a marked degree of pinwheeling with an S3 THV in the 23 mm SAPIEN XT. Pinwheeling is a result of redundant leaflet issue and may have implications for long-term leaflet function and durability. A high degree of pinwheeling can lead to excessive strain on THV leaflets that may accelerate wear or increase the risk of valve thrombosis.
Limitations
Bench testing may not entirely reflect how a THV will expand in a patient’s native annulus, within a degenerated transcatheter heart valve or when deployed under physiological conditions. Redo TAVI was performed in new THVs; however, performance and anchoring may vary in a degenerated THV. The impact of redo TAVI on coronary obstruction and coronary access was not evaluated in this study. Acquisition of THVs for the purposes of independent bench testing is challenging and not all THV designs, sizes, or implantation positioning could be assessed in this study. Valves that were considered “failed” valves were older-generation THVs and are not currently available contemporary iterations. In the case of implantation of the ALLEGRA into a 29 mm SAPIEN XT, the large size of these valves was not available. The relationship of redo TAVI samples to coronary height and the potential for sinotubular junction (STJ) sequestration were beyond the scope of this study and were not assessed.
Conclusions
This study demonstrates that most THV designs and implantation depths result in favourable performance following redo TAVI. Whether the leaflets of a failed THV are intra- or supra-annular may have important implications for the positioning and appropriate size selection of a redo THV implant. Certain THV designs or implantation positions may be more desirable when performing redo TAVI.
|
Impact on daily practice As the use of TAVI expands to younger patients with a longer life expectancy, there is a higher likelihood that repeat intervention will be required. Redo TAVI is a potential treatment option. This study demonstrates that implanters must consider the design of the index failed THV and the impact of different THV designs and implantation positions on redo TAVI. Considering these factors will help ensure optimal THV performance following redo TAVI. |
Conflict of interest statement
P. Blanke and J. Leipsic are consultants to Edwards Lifesciences and provide CT core lab services for Edwards Lifesciences, Medtronic, Neovasc, Guided Delivery Systems, and Abbott, for which no direct compensation is received. P. Blanke is a consultant to Abbott, Neovasc and Circle Cardiovascular Imaging. D. Frank is a consultant to Edwards Lifesciences and Medtronic and has received research funding from Edwards Lifesciences. J.G. Webb is a consultant to, and has received research funding from, Edwards Lifesciences, Abbott, and ViVitro Labs. J. Sathananthan is a consultant to Edwards Lifesciences and Medtronic. L. Søndergaard has received consultant fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis. D. Wood is a consultant to, and has received research funding from, Edwards Lifesciences and Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Redo TAVI with low implantation of a 26 mm S3 into a 29 mm Evolut R demonstrating cycle-to-cycle variation with impaired S3 leaflet coaptation.

