Abstract
Background: Coronary obstruction and access are concerns in patients undergoing redo transcatheter aortic valve implantation (TAVI).
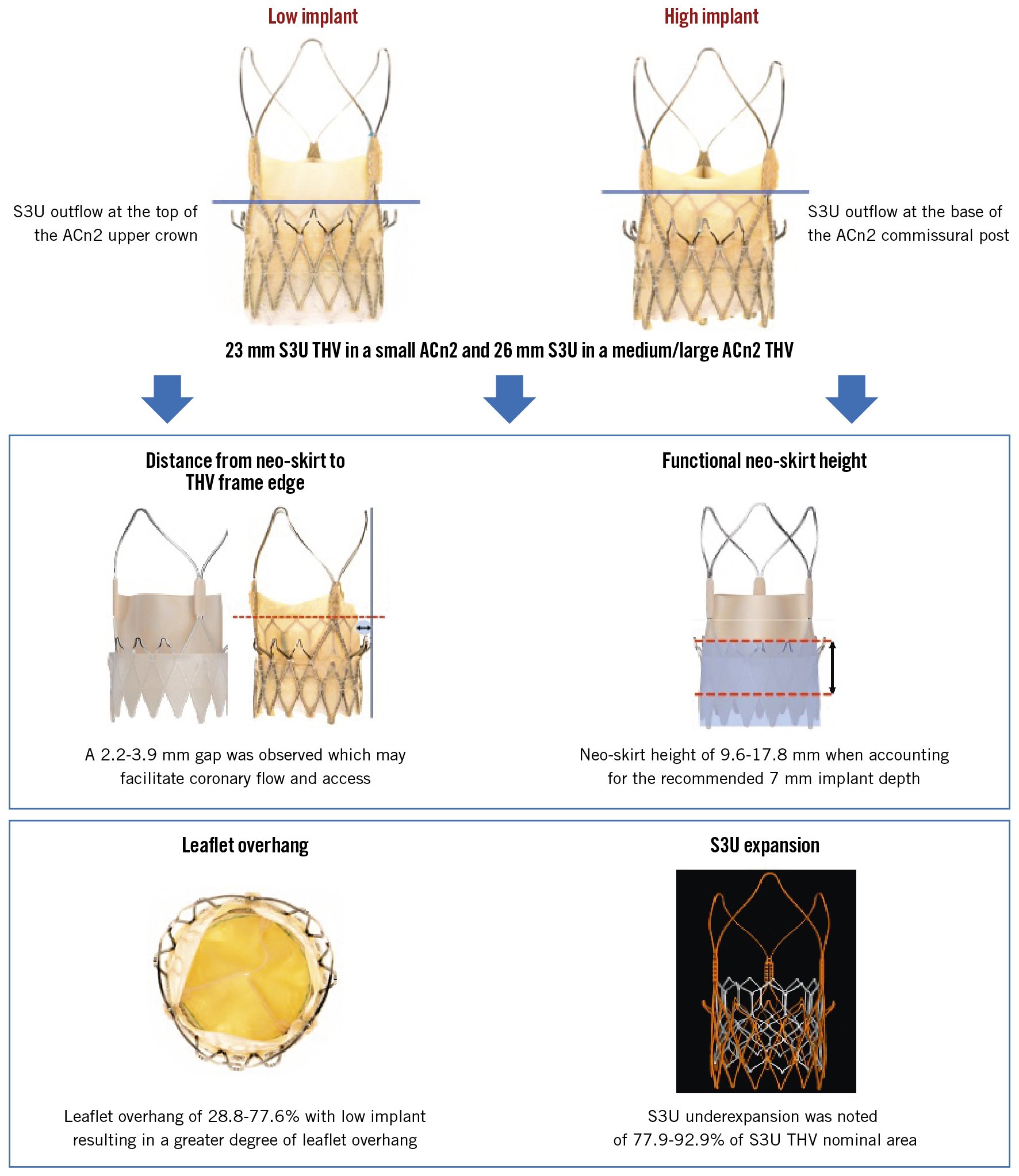
Aims: We sought to assess the neoskirt height, leaflet overhang, leaflet deflection,and transcatheter heart valve (THV) expansion and performance, at 2 different implant depths, of the SAPIEN 3 Ultra (S3U) within the ACURATE neo2 (ACn2) THV.
Methods: An in vitro study was performed with a 23 mm S3U deployed within a small (S) ACn2 and a 26 mm S3U deployed within a medium (M) and a large (L) ACn2. The S3U outflow was positioned at the top of the ACn2 crown (low implant) and at the base of the commissural post of the ACn2 (high implant). Testing was performed under physiological conditions as per ISO-5840-3 standard.
Results: The neoskirt height was shorter when the S3U outflow was positioned at a low implantation depth (S: 9.6 mm, M: 12.2 mm, L: 13.8 mm vs S: 15.2 mm, M: 15.1 mm, L: 17.8 mm ACn2 for high implants). Hydrodynamic performance was acceptable for all configurations. Leaflet overhang was <50% for all configurations except the low implant of the 26 mm S3U in the L ACn2 (77.6%). There was a gap from the side of the neoskirt to the outer border of the THV frame which was >2 mm for all configurations. The S3U was underexpanded for all configurations, and the achieved area was 77.9%-92.9% of the expected nominal area.
Conclusions: Redo TAVI with an S3U within an ACn2 has favourable hydrodynamics and moderate leaflet overhang. Importantly, the design of the ACn2 results in a neoskirt that is not deflected all the way to the outer dimensions of the THV, hence creating a space that facilitates coronary flow and access.
Introduction
Transcatheter heart valves (THV) may fail and repeat intervention may be required12. Redo transcatheter aortic valve implantation (TAVI), when feasible, has been shown to be an acceptable treatment for THV failure34. There are several considerations when performing redo TAVI, including the implant depth of the index THV, the index THV expansion, leaflet overhang and the neoskirt height5. Importantly, different failed THV designs may have unique technical considerations in order to optimise results following redo TAVI. There is currently limited clinical experience to guide THV choice and implant position. In lieu of limited clinical data, bench testing can provide insights.
A concern with redo TAVI is the creation of a neoskirt, where the leaflets of the failed valve are pushed outward by the new THV, creating a tube graft which may lead to coronary obstruction or impede future coronary access56. Neoskirt heights are typically greater in tall-frame THV with supra-annular leaflets but may vary across different supra-annular THV designs. The degree of leaflet deflection, which refers to the distance from the neoskirt to the edge of the THV frame, may also vary across THV designs. A THV design where the leaflets are not maximally deflected to the edge of the THV frame may mitigate coronary obstruction.
A recent study showed that a low SAPIEN 3 (S3; Edwards Lifesciences) THV implant within a failed Evolut R (Medtronic) results in a lower neoskirt height without compromising the THV hydrodynamic performance, despite a higher degree of leaflet overhang5. The ACURATE neo2 (ACn2) is another commercially available tall-frame valve that has unique design features where the upper segment of the valve has an incomplete frame with 3 stabilisation arches. There is an upper crown which is designed to push down on the native leaflets7. Redo TAVI in the ACn2 is poorly understood.
We performed an in vitro study to assess valve performance, functional neoskirt height, leaflet overhang, leaflet deflection, THV expansion and hydrodynamic performance when implanting an S3 Ultra (S3U) THV within an ACn2 THV at different implantation positions.
Methods
Study methods
In vitro testing was performed in collaboration with the Cardiovascular Translational Laboratory (Vancouver, Canada) and Boston Scientific (Galway, Ireland). This study was performed under physiologic test conditions with no human or animal participants, and ethics approval was not required.
Valves
The ACn2 was used as the index “failed” valve, and small (S), medium (M) and large (L) ACn2 sizes were evaluated (3 ACn2 valves in total). The ACn2 THV incorporates a self-expanding nitinol frame, supra-annular leaflets and an active PV seal which is 60% larger than the prior-generation ACURATE neo (ACn) valve7. Compared to the previous ACn THV, the ACn2 is designed with a lower commissural post leaflet attachment (3 mm lower for the S and M, and 2 mm lower for the L ACn2) and scalloped leaflets with a shorter-than-expected neoskirt. The upper segment of the valve has an incomplete frame with 3 stabilisation arches, and the upper crown is designed to push down on the native leaflets. The edge of the upper crown represents the outer edge of the THV frame. The height of the ACn2 across the sizes ranges between 48 and 51 mm. The ACn2 waist diameters are 23 mm, 25 mm and 27 mm for the S, M and L THV, respectively. The ACn2 is designed to be routinely implanted at a 7 mm implantation depth. This gives an effective skirt height of 8 mm above the annular plane, as the total skirt height is 15 mm for all sizes7. The latest-generation S3U device was designed to allow a larger area of surface contact with the native valve (up to 50%) with a higher polyethylene terephthalate outer skirt (>40% higher compared to the S3 device)8.
Redo TAVI configurations
Redo TAVI configurations were performed using a 23 mm S3U within an S ACn2, a 26 mm S3U within an M ACn2, and a 26 mm S3U within an L ACn2 (3 S3U valves in total). The S3U was implanted at 2 different positions for each size configuration. Positioning was based on the S3U outflow to either the 1) top of the upper crown as a low position, or 2) to the base of the marker of the stabilisation arches as a high position (Figure 1). All S3U valves were deployed with nominal volume per manufacturer recommendations, and new valves were used for low and high implants in the same redo TAVI configurations.
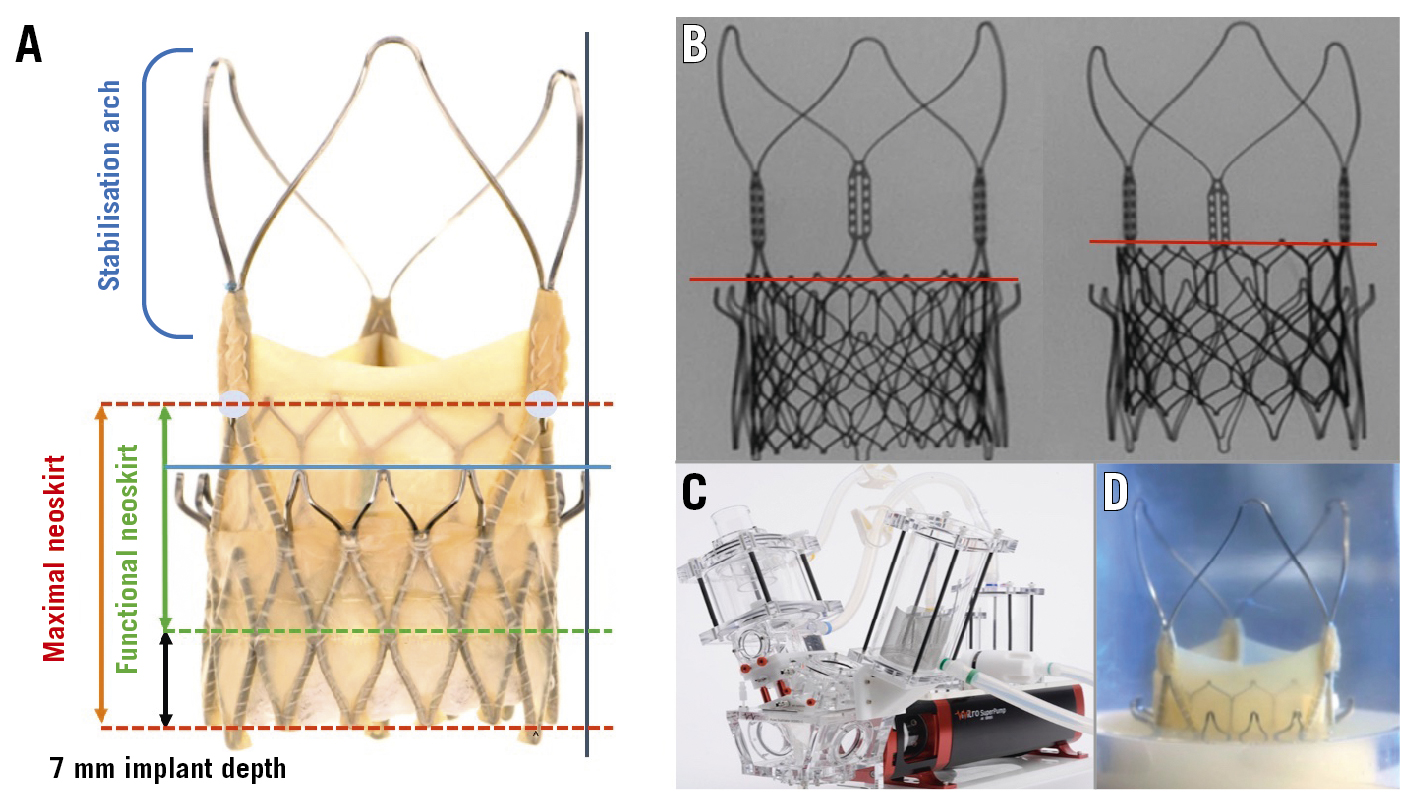
Figure 1. Study methodology. A) ACn2 landmarks (blue line: top of the ACn2 upper crown, blue circles: base of the ACn2 commissural tab), maximal neoskirt height (red lines and arrow); functional neoskirt height measurements (red and green lines, green arrow) subtracting 7 mm based on manufacturer-recommended implantation depth, and edge of the THV frame (black vertical line); B) S3U at the 2 implantation positions based on S3U outflow at the top of the ACn2 crowns (low implant) and at the base of the ACn2 commissural tab (high implant); C) Vivitro pulse duplicator for hydrodynamic assessment and (D) ACn2 THV example positioned in a holder for testing. ACn2: ACURATE neo2; S3U: SAPIEN 3 Ultra; THV: transcatheter heart valve
neoskirt and functional neoskirt
The neoskirt height was defined as the distance between the ACn2 THV inflow and the pinned leaflet-free edge height of the ACn2 THV at the outflow of the S3U THV. The neoskirt height was determined using the Smartscope (OGP) vision-based measurement system. As the ACn2 is designed to be implanted routinely at a depth of 7 mm, the functional neoskirt height was measured by subtracting 7 mm from the total neoskirt as this portion of the valve would be positioned in the left ventricular outflow tract, below the annular plane, and hence pose no risk to coronary obstruction. The functional neoskirt height was calculated for each configuration at each implantation depth.
Leaflet overhang
The percentage of leaflet overhang was defined as the percentage of orifice blockage due to the inward flexing of the unpinned portion of the ACn2 THV leaflets during the diastolic phase (Figure 2A). The baseline orifice area (i.e., inner diameter at the base of index valve) was traced using ImageJ software (National Institutes of Health Image) of the still diastolic images obtained from the hydrodynamic test videos using the “polygon selections” tool within the software and displayed in mm by setting the scale to a known distance and calculating it using the “analyse/measure” tool. The leaflet overhang orifice area was determined by tracing the flexed leaflet edge of an index valve and determining the orifice area. The derived leaflet area was defined as follows: the baseline orifice area − the leaflet overhang orifice area. The % leaflet overhang was defined as the following: the derived leaflet area/baseline orifice area x 100.
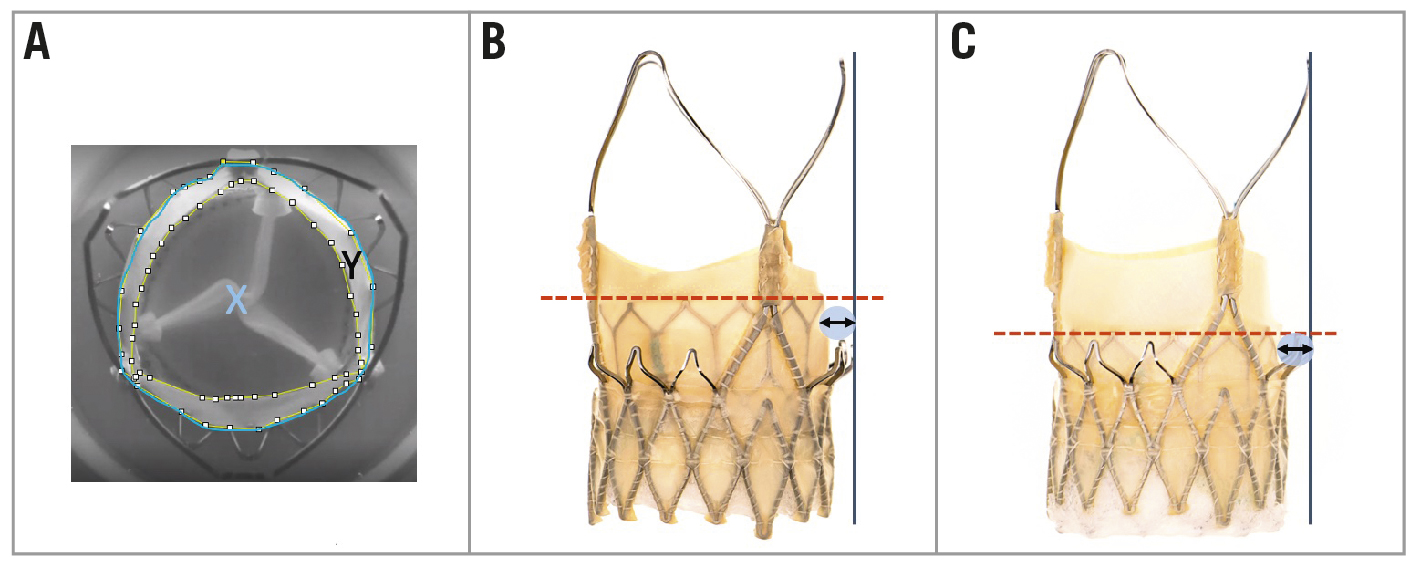
Figure 2. Leaflet overhang and leaflet deflection. A) Example of measurements made to determine the degree of leaflet overhang (%) of the “failed” index THV leaflets (X=total area in blue, Y=area between the white dots; leaflet overhang=(Y/X)*100); B) Distance (black arrow) from top of the neoskirt (red line) to the edge of the ACn2 THV frame in the high S3U implant position;C) Distance (black arrow) from top of the neoskirt (red line) to the edge of the ACn2 THV frame in the low S3U implant position. ACn2: ACURATE neo2; S3U: SAPIEN 3 Ultra; THV: transcatheter heart valve
Degree of leaflet deflection relative to THV frame
In some configurations of redo TAVI the leaflets may not be deflected up to the outer border of the THV frame. This phenomenon was defined by outlining the outer border of the ACn2 (edge of the upper crown) and calculating the distance and the area between this border and the neoskirt. Area measurements are reported in mm2 and distance in mm (Figure 2B, Figure 2C).
Hydrodynamic assessment
A pulse duplicator test system (Vivitro Laboratories) was used to assess hydrodynamic performance for all redo TAVI configurations. The index ACn2 THV were placed in a holder fabricated from silicone (Figure 1). The holder compliance was chosen in accordance with ISO 5840-3:20219. The ACn2 valves were fixed in the gaskets, which had diameters that were representative of the upper limit of the respective indicated patient annular range per ACn2 instructions for use (i.e., the ACn2 S was deployed in a 22 mm diameter gasket, the ACn2 M was deployed in a 24 mm diameter gasket, and the ACn2 L was deployed in a 26 mm diameter gasket). The lowest interference between the 2 THV was chosen because it was deemed to be the worst case scenario for inter-THV leakage and stability. The test medium used for this study was 40% glycerol containing saline solution with a target viscosity of 3.8 centipoise to approximate the viscosity of blood. Results were taken for 20 consecutive cycles, repeated 3 times and then averaged. High-speed video filming was performed to assess the index valve kinematics from the outflow and inflow ends. Pulsatile forward flow performance was measured at a nominal beat rate of 70±1 beats/min, systolic duration of 35±5%, mean aortic pressure of 100±2 mmHg, and simulated cardiac output of 5.0±0.1 L/min. The mean gradient (mmHg), regurgitant fraction (%), and effective orifice area (EOA; cm2) were assessed. The maximum allowable regurgitant fraction in accordance with ISO 5840-3 is <20%. The total regurgitant fraction, which included closing and intervalvular regurgitation post-S3 closure, was assessed. The EOA was computed according to a simplified version of the Bernoulli equation, as previously described in ISO 58409.
ACn2 and SAPIEN 3 Ultra frame expansion after redo TAVI
The baseline diameter of the index ACn2 THV were measured at the level of the inflow, mid-portion and S3U outflow pinning positions to assess the risk of sinus sequestration and potential contact with the sinotubular junction. The change in radii was reported to maintain consistency with the “valve-to-coronary” measurements being taken as part of the patient screening process.
The S3U area and diameter after nominal inflation were measured by micro-computed tomography at the level of the inflow, mid-portion and S3U outflow pinning positions to assess the S3U expansion when implanted within the ACn2 THV.
The expanded diameter and increase in radius after redo TAVI were assessed for all S3U THV within ACn2 THV configurations. ACn2 and S3U THV diameters were measured using an Optical Comparator (Micro-Vu) for measurement.
Protocol imaging
Multimodality imaging, including fluoroscopy, was performed using the General Electric OEC 9900 Elite Mobile C-arm X-ray system, and micro-computed tomography and high-resolution photography were performed for each THV at each implantation configuration with the Keyence Digital Microscope. Micro-computed tomography was performed using a continuous Cone Beam quick scan to reduce dehydration of the valve tissue including 1,200 projections with 3.98 magnification, 536,365 mm focus detector distance and 135,000 mm focus object distance with a voltage current of 110 kV and a current of 200.0 μA. Frame rate detector exposure was 5 Hz with an exposure time of 200,000 ms. VGSTUDIO MAX (Volume Graphics) was used to analyse, segment and measure the computed tomography reconstructed data of each of these redo TAVI configurations.
High-resolution photography was performed at a prespecified magnification and a fixed camera height. High-speed video from hydrodynamic testing was also performed510.
Statistics
The radii increase, diameter increase and leaflet pinning heights, also referred to as neoskirt heights, are expressed in mm. The index leaflet overhang is reported as a percentage relative to baseline orifice. The hydrodynamic variables, EOA, pressure gradients, and total regurgitation fraction are reported as mean±standard deviation (SD).
Results
Functional neoskirt
The S3U THV implanted within the ACn2 THV resulted in functional neoskirt heights ranging between 9.6 mm and 17.8 mm (Table 1). The functional neoskirt heights were shortest in the low implantation positions, with the lowest value (9.6 mm) for the 23 mm S3U THV in the S ACn2 THV. Higher functional neoskirt heights were noted in the high implantation position, with the highest value (17.8 mm) obtained for the 26 mm S3U THV implanted in the L ACn2 THV. A lower implant position can reduce the functional neoskirt height by as much as 8.2 mm as compared to high implantation (Figure 3, Figure 4, Central illustration).
Table 1. Neoskirt height, leaflet overhang and leaflet deflection with variable implant depths.
| ACn2 | S3U | Implant depth (S3 outflow) | Neoskirt height (mm) | Functional neoskirt (mm) | Leaflet overhang (%) | Leaflet deflection (mm) | Leaflet deflection area (mm2) |
|---|---|---|---|---|---|---|---|
| S | 23 mm | Low | 16.6 | 9.6 | 34.7 | 2.7 | 6.8 |
| High | 22.2 | 15.2 | 28.8 | 3.9 | 34.4 | ||
| M | 26 mm | Low | 19.2 | 12.2 | 48.1 | 2.7 | 13.8 |
| High | 22.1 | 15.1 | 30.1 | 2.3 | 22.8 | ||
| L | 26 mm | Low | 20.5 | 13.5 | 77.6 | 2.6 | 15.1 |
| High | 24.8 | 17.8 | 27.6 | 2.2 | 9.5 | ||
| Note: 0% overhang is indicative of full pinning of ACn2 index leaflets. The functional neoskirt height was measured by subtracting 7 mm from the total neoskirt height as the ACn2 is designed to be implanted routinely at an implanted depth of 7 mm, this portion of the valve would be positioned in the left ventricular outflow tract and hence pose no risk to coronary obstruction. ACn2: ACURATE neo2; L: large; M: medium; S: small; S3U: SAPIEN 3 Ultra | |||||||
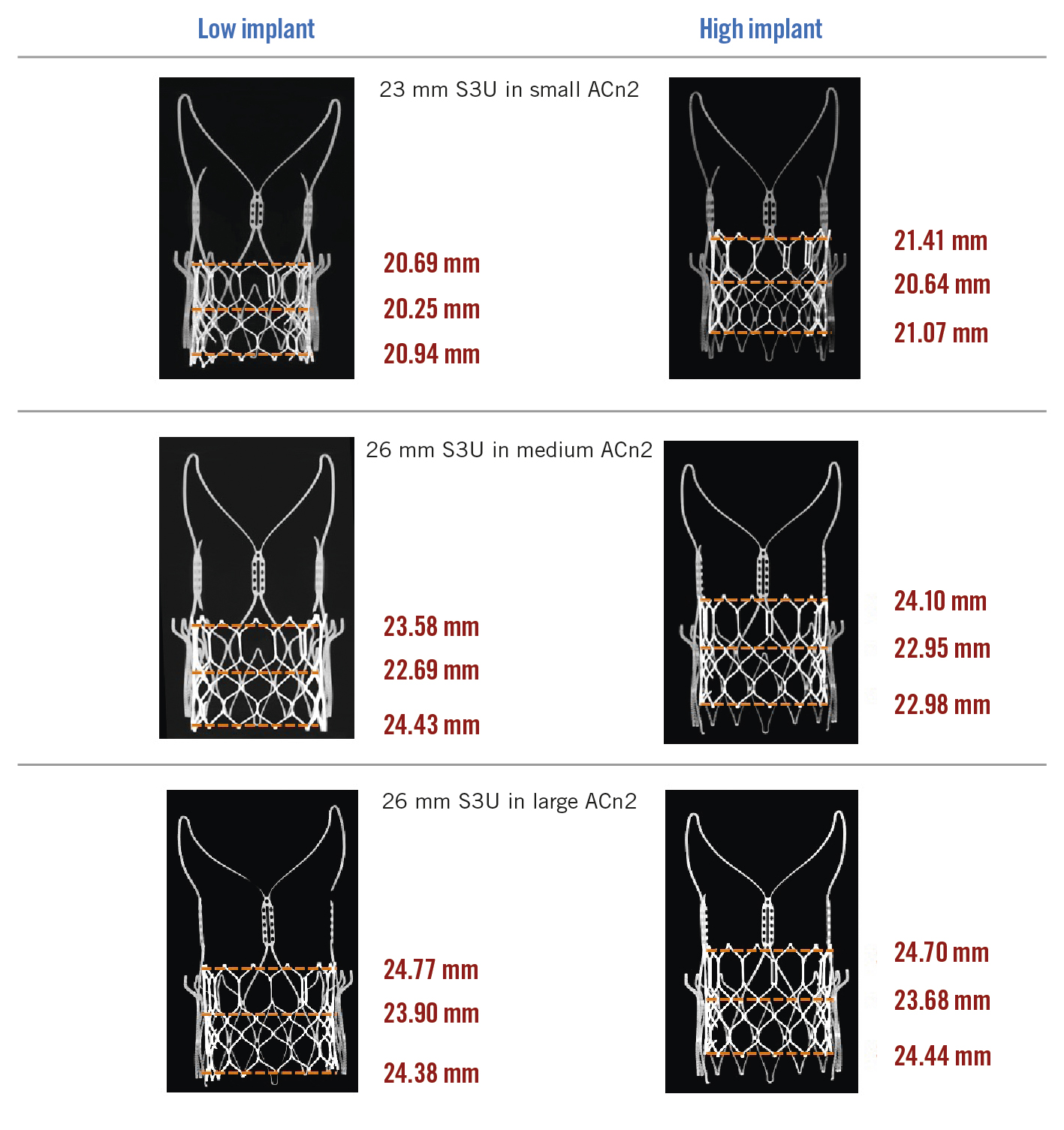
Figure 3. S3U THV diameter expansion at the outflow, midportion and inflow at different implant positions for each configuration. ACn2: ACURATE neo2; S3U: SAPIEN 3 Ultra; THV: transcatheter heart valve
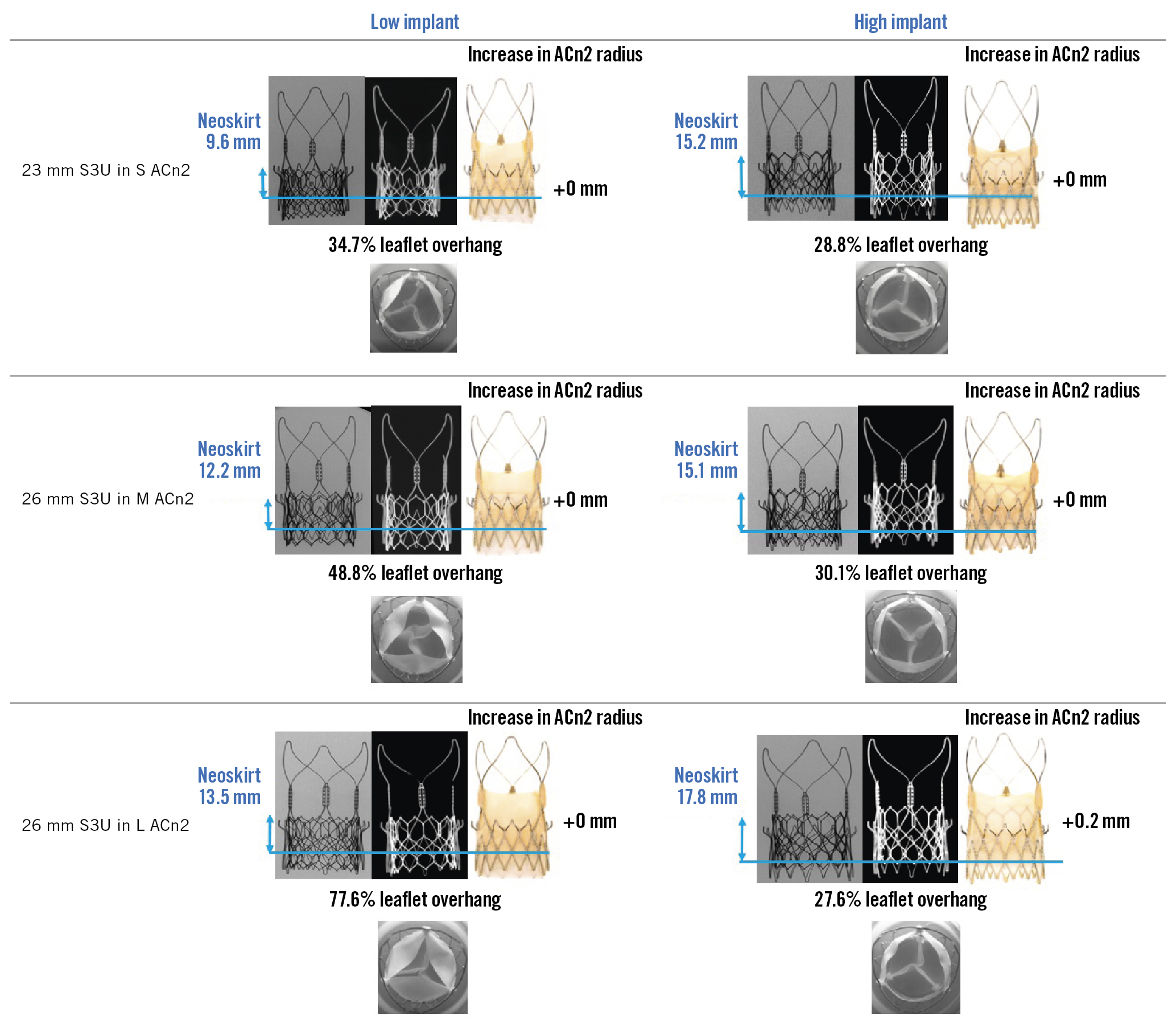
Figure 4. Redo TAVI test configurations of different-sized SAPIEN 3 Ultra within ACn2 THV. The neoskirt height, degree of leaflet overhang (%) and increase in the ACn2 radius (mm) after redo TAVI with SAPIEN 3 Ultra in ACn2 for each THV size are outlined. ACn2: ACURATE neo2; S3U: SAPIEN 3 Ultra; THV: transcatheter heart valve
Central illustration. Technical implications of redo-TAVI with the SAPIEN 3 Ultra in ACn2 THV at different implant positions. ACn2: ACURATE neo2; S3U: SAPIEN 3 Ultra; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
Leaflet overhang
The degree of leaflet overhang and impact on leaflet kinematics had minimal variation by the S3U THV implant position within the ACn2 THV except for the low implant of the 26 mm S3U within the L ACn2 (Table 1, Figure 3, Figure 4). Leaflet overhang ranged from 28.8% to 77.6% across various implant positions (Central illustration). Greater leaflet overhang was observed when the S3U THV outflow was implanted low within the ACn2 THV, and higher S3U implants within the ACn2 THV reduced the degree of leaflet overhang. Similar and mild degrees of leaflet overhang across the different THV size combinations were highlighted in high implants.
Degree of leaflet deflection relative to THV frame
The distance from the neoskirt to the outer edge of the THV frame ranged from 2.2 mm for the high implant of the 26 mm S3U in the M ACn2 THV to 3.9 mm for the high implant of the 23 mm S3U in the S ACn2 THV (Table 1, Central illustration). The area of leaflet deflection relative to the THV frame ranged from 6.8 mm2 for the 23 mm S3U placed low in the S ACn2 THV to 34.4 mm2 for the high implant of the 23 mm S3U in the S ACn2 THV.
Hydrodynamic assessment
Hydrodynamic performances were within accepted standards as per ISO 5840-3 guidelines, with the mean gradient being slightly lower in the high S3U implant. A regurgitant fraction <20% was demonstrated for all tested configurations (Table 2).
Table 2. Functional performance of SAPIEN 3 Ultra THV at variable implant depths.
| ACn2 | S3U | Implant depth | EOA (cm2) | Mean gradient (mmHg) | Total regurgitant fraction (%) |
|---|---|---|---|---|---|
| S | 23 mm | Low | 1.9±0.02 | 16.7±0.3 | 14.9±0.7 |
| High | 1.9±0.01 | 15.1±0.2 | 12.7±0.5 | ||
| M | 26 mm | Low | 2.3±0.01 | 11.5±0.1 | 17.3±0.7 |
| High | 2.3±0.01 | 11.2±0.08 | 15.1±0.6 | ||
| L | 26 mm | Low | 2.5±0.01 | 11.1±0.08 | 19.4±0.5 |
| High | 2.5±0.02 | 10.7±0.2 | 17.2±0.4 | ||
| Note: Acceptable EOA values for certain annular diameters have been linear interpolated from ISO 5840-3:2021 specifications using adjacent deployed valve diameter. ACn2: ACURATE neo2; EOA: effective orifice area; L: large; M: medium; S: small; S3U: SAPIEN 3 Ultra | |||||
ACn2 and SAPIEN 3 Ultra frame expansion after redo TAVI
The increase in the dimensions of the ACn2 THV after S3U THV deployment are found in Supplementary Table 1, with a maximal increase in radius of 0.2 mm across tested configurations.
S3U diameter expansion at the inflow, mid-portion and the outflow are highlighted in Supplementary Table 2. The S3U THV was underexpanded for all THV configurations, predominantly at the mid-portion. S3U THV underexpansion was more significant for the 26 mm S3U in the M ACn2 at the mid-portion for the low implant position (77.9%). S3U THV area expansion was better for the 26 mm S3U in the L ACn2 at the outflow in the high implant position (92.9%).
Discussion
There is currently limited guidance or clinical experience on THV choice or positioning when performing redo TAVI. In a tall frame, where the leaflets are in a supra-annular position, there may be increased concern for coronary obstruction, given the potential tall neoskirt that is created following placement of the new THV. However, not all tall-frame valves necessarily carry the same risk. In the case of a failed ACn2, redo TAVI with another supra-annular THV may potentially, in some cases, result in a taller neoskirt, increasing the risk of coronary obstruction and could lead to challenging coronary access5. This approach is likely undesirable for many patients. However, use of a short-frame valve for the redo THV may mitigate these risks. In this study we evaluated the use of an S3U THV positioned within an ACn2 THV as a potential treatment method for a failed ACn2 THV. We demonstrated that this strategy was associated with a favourable neoskirt height and hydrodynamics for most configurations, despite the presence of leaflet overhang. Some considerations for the ACn2 which may potentially facilitate redo TAVI with an S3U and mitigate coronary obstruction are 1) the initial implant depth of the failed ACn2, and 2) the degree of leaflet deflection relative to the THV frame.
The ACn2, as per manufacturer recommendations, has a target depth of 7 mm routinely for all cases in the 3-cusp coplanar view. Unlike other self-expanding valves, such as in the Evolut Platform for which a high implant is recommended, a higher implant is not recommended for the ACn2. Prior bench studies of neoskirt heights across different THV platforms have measured the neoskirt height from the inflow of the valve to the top of the pinned leaflet56. This technique was adopted to maintain a consistent technique but is limited as it does not account for the implant depth of the THV. In the case of the ACn2, the functional neoskirt is shorter when considering a standard implant depth of 7 mm. In addition, the degree of leaflet deflection relative to the THV frame also has an impact on the risk of coronary obstruction. Most THV have a complete frame, and the leaflet is deflected all the way to the frame, forming the neoskirt. However, the ACn2 is designed with an incomplete frame and an upper crown which marks what could be considered the edge of the THV frame7. Redo TAVI in the ACn2 with the S3U results in a neoskirt that does not extend all the way to the end of the upper crown, and hence this creates a gap which would help mitigate the risk of coronary obstruction and facilitate future coronary access.
When performing redo TAVI using an S3U THV in an index ACn2 THV, aligning the S3U outflow to landmarks on the index THV frame will provide a better method to determine the expected neoskirt height. Moreover, as previously described, alignment according to the THV outflow is more predictable, as the S3U THV is foreshortened most appreciably from the inflow side5. Implanters may wish to tailor their implant position to balance the degree of leaflet overhang and the neoskirt height. In this study, the position of the S3U THV in an index ACn2 THV had a moderate impact on leaflet overhang. In particular, the degree of leaflet overhang was <50% for all configurations except for the low implant of the 26 mm S3U in the L ACn2. In comparison, a similar analysis demonstrated severe leaflet overhang (up to 94%) when an S3 was implanted low in an Evolut THV5. While this study was performed using new THV, it could be anticipated that similar results can be achieved in patients without significant calcification. However, in severely degenerated leaflets with a stenotic mechanism, residual new outflow obstruction may occur in the case of a low implant of the new THV with significant leaflet overhang as well as the potential for thrombosis in cases of impaired flow.
We found that lower implantation of a short-frame THV within an ACn2 resulted in a reduction in the functional neoskirt height.
In the present study, micro-computed tomography measurements demonstrated that the S3U was underexpanded for all configurations when implanted within the ACn2, irrespective of the implant position. The mid-segment of the THV was more likely to be underexpanded. S3U THV have been shown to be underexpanded in routine valve-in-valve or native anatomy11. However, THV underexpansion may result in a range of leaflet pinwheeling and impact THV function and durability1011. The impact of THV underexpansion on the risk of hypoattenuated leaflet thickening remains unknown to date although THV underexpansion has been shown to be associated with blood stasis in experimental models12. Late valvuloplasty, as a staged procedure, has been shown to be feasible in native annulus and valve-in-valve configurations to improve THV expansion. However, post-dilatation of 2 THV metallic frames following redo TAVI may be challenging and may not allow further THV expansion11. Thus, the final degree of required oversizing of the new THV has to be addressed when considering redo TAVI and may be based on individual patient anatomy.
Ultimately, there is no ideal THV design or implant position that would be considered optimal for all patients. Rather, several factors will influence redo TAVI including patient anatomy, the position of the failed index THV and the choice of the new THV design. All these factors must be considered when considering redo TAVI in a failed supra-annular THV. As TAVI continues to expand in younger patients, it will be even more important to determine the optimal treatment modality in the context of a patient’s lifetime and anticipated life expectancy1314. Heart Teams must carefully consider appropriate treatment options (TAVI vs surgery), THV type and implant technique in these patients1516. Anatomic factors and index THV choice may preclude some patients from future redo TAVI, and these patients may be better managed with TAVI explant and surgery121718. The index THV choice and feasibility of future redo TAVI will be crucial for Heart Teams to consider at the time of the first procedure.
Study limitations
Bench testing design may not reflect physiological conditions in clinical practice. Indeed, the index valves were new, and hence in a degenerated in vivo failed valve which is calcified, the leaflet overhang and the paravalvular leak, as well as the mean gradient, may vary compared to the present study. Similarly, in vivo expansion of the index THV may vary compared to a bench model, and intervalvular leak between the 2 THV may vary following redo TAVI. Additionally, a holder was used to simulate the anatomy but may not reflect patient anatomy with elliptical annuli and did not allow an assessment of THV expansion at the level of the holder (ACn2 inflow). While the majority of configurations had favourable hydrodynamic performance, leaflet overhang and neoskirt height may have important short- and long-term clinical implications which were not assessed in this study. In vivo, leaflet overhang may lead to THV dysfunction and impaired neo-sinus flow with a potential impact on redo THV degeneration and thrombosis. Finally, all data were provided assuming a 7 mm implantation depth of the ACn2 THV. In clinical practice, the implant depth may vary and potentially may be higher, which would slightly increase the neoskirt height compared to what is described in this study. Additionally, in the case of the medium ACn2, a 23 mm S3U could have been implanted instead of a 26 mm S3U. Unfortunately, this configuration could not be tested in this study due to challenges with acquiring THV for bench testing. Use of a smaller 23 mm S3U in a medium ACn2 could have led to less underexpansion of the S3U.
Conclusions
Redo TAVI with an S3U within an index ACn2 has favourable S3U THV hydrodynamics and mild to moderate leaflet overhang. Importantly, the unique design of the ACn2 results in a neoskirt that is not deflected all the way to the outer dimensions of the lower crown, hence creating a space that may better preserve coronary flow and access. The impact of S3U frame underexpansion and leaflet overhang in the low implant position on valve haemodynamics and durability will need further clinical evaluation.
Impact on daily practice
Placement of an S3U THV within an index ACn2 THV results in an acceptable functional neoskirt height with no significant compromise to the S3U valve function and mild-to-moderate leaflet overhang, regardless of the implant depth. The S3U THV was routinely underexpanded. The long-term implications of redo TAVI on THV performance and durability will be assessed in clinical studies. The feasibility of coronary access and impact of THV underexpansion following redo TAVI will also be assessed in future studies.
Conflict of interest statement
M. Akodad has received research funding from Medtronic, Biotronik, MUSE Explore, and Federation Française de Cardiologie. D. Meier is supported by the Swiss National Science Foundation (grant P2LAP3_199561). S. Sellers is a consultant for Providence Health Care, Edwards Lifesciences, and Medtronic. O. De Backer received research grants from Boston Scientific. D. Mylotte is consultant for Boston Scientific, MDT, and MicroPort. C. Frawley is an employee of Boston Scientific in research and development engineering. L. Lynch is an employee of Boston Scientific in research and development engineering. G. Tang is a physician proctor and consultant for Medtronic; a consultant and physician advisory board member for Abbott Structural Heart; a consultant for NeoChord; and physician advisory board member for JenaValve. D. Wood is a consultant and receives unrestricted grant support from Medtronic, Edwards Lifesciences, and Abbott Vascular. L. Sondergaard has received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT. J.G. Webb is a consultant for Edwards Lifesciences. J. Sathananthan is a consultant for Edwards Lifesciences, Medtronic, and Boston Scientific; and has received speaking fees from Edwards Lifesciences and NVT. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

