Abstract
BACKGROUND: There are limited data regarding treatment for failed balloon-expandable transcatheter heart valves (THVs) in redo-transcatheter aortic valve implantation (TAVI).
AIMS: We aimed to assess THV performance, neoskirt height and expansion when performing redo-TAVI with the ACURATE platform inside a SAPIEN 3 (S3) compared to redo-TAVI with an S3 in an S3.
METHODS: Redo-TAVI was performed on the bench using each available size of the S3, the ACURATE neo2 (ACn2) and the next-generation ACURATE Prime XL (AC XL) implanted at 2 different depths within 20 mm/23 mm/26 mm/29 mm S3s serving as the “failed” index THV. Hydrodynamic testing was performed to assess THV function. Multimodality assessment was performed using photography, X-ray, microcomputed tomography (micro-CT), and high-speed videos.
RESULTS: The ACURATE in S3 combinations had favourable hydrodynamic performance compared to the S3 in S3 for all size combinations. In the 20 mm S3, redo-TAVI with the ACn2 had lower gradients compared to the S3 (mean gradient 16.3 mmHg for the ACn2 vs 24.7 mmHg for the 20 mm S3 in 20 mm S3). Pinwheeling was less marked for the ACURATE THVs than for the S3s. On micro-CT, the S3s used for redo-TAVI were underexpanded across all sizes. This was also observed for the ACURATE platform, but to a lesser extent.
CONCLUSIONS: Redo-TAVI with an ACn2/AC XL within an S3 has favourable hydrodynamic performance and less pinwheeling compared to an S3 in S3. This comes at the price of a taller neoskirt.
Transcatheter aortic valve implantation (TAVI) is increasingly being performed in younger patients and those with longer anticipated longevity, thus there is a higher likelihood of valve degeneration and need for repeat intervention. In the USA, almost 50% of patients <65 years of age with isolated severe aortic stenosis were treated with TAVI in 20211. Moreover, at least a third of patients undergoing TAVI aged <75 years old are anticipated to live beyond 10 years2. Given the potential for transcatheter heart valve degeneration over time, it is anticipated that the number of degenerated transcatheter heart valves (THVs) requiring reintervention will increase in the future. Redo-TAVI is a potential treatment option for these patients, and early experience has demonstrated that this approach is safe and feasible in patients at low risk of coronary obstruction. However, beyond acute procedural success, there are limited data available to guide procedural optimisation, device selection and optimal long-term haemodynamic performance3. Given the number of commercially available THV devices, there are several potential redo-TAVI combinations, and it is unlikely that clinical data alone will answer these unaddressed issues. Bench testing has provided novel insights related to redo-TAVI, which include variations in neoskirt height, THV expansion, leaflet coaptation, leaflet overhang and haemodynamic performance. Importantly, each potential redo-TAVI combination is unique and has its own technical considerations and potential issues45. Additionally, the expansion of the index THV is influenced by the size of the native annulus, and this will impact the choice of type and size of the redo THV.
The ACURATE neo2 (ACn2; Boston Scientific) is a commercially available supra-annular valve with a unique design feature where the upper segment of the valve is largely open with an incomplete frame with 3 stabilisation arches. This offers the haemodynamic advantage of a supra-annular device while preserving options for future coronary access. To date, little is known about performance and optimal deployment conditions when performing redo-TAVI with the ACn2. Additionally, the next generation of the ACn2, the ACURATE Prime XL (AC XL; Boston Scientific) has been developed, allowing treatment of larger annular ranges.
We performed an in vitro study to assess the optimal position and performance when implanting an ACn2 or an AC XL within a SAPIEN 3 (S3; Edwards Lifesciences) THV compared to using an S3 in S3 redo-TAVI combination.
Methods
OBJECTIVES
The present study had the following objectives:
1) To assess THV hydrodynamic performance, neoskirt height, pinwheeling and expansion when performing redo-TAVI with an ACn2/AC XL inside an S3 versus redo-TAVI with an S3 inside an S3.
2) To assess the impact of annular size on THV performance when performing redo-TAVI with the ACn2/AC XL inside an S3 versus an S3 in S3.
STUDY METHODS
In vitro testing was performed in collaboration with the Cardiovascular Translational Laboratory (Vancouver, Canada) and Boston Scientific (Galway, Ireland). This study was performed under physiological test conditions with no human or animal participants, and ethics approval was not required. Study methods are summarised in Figure 1.
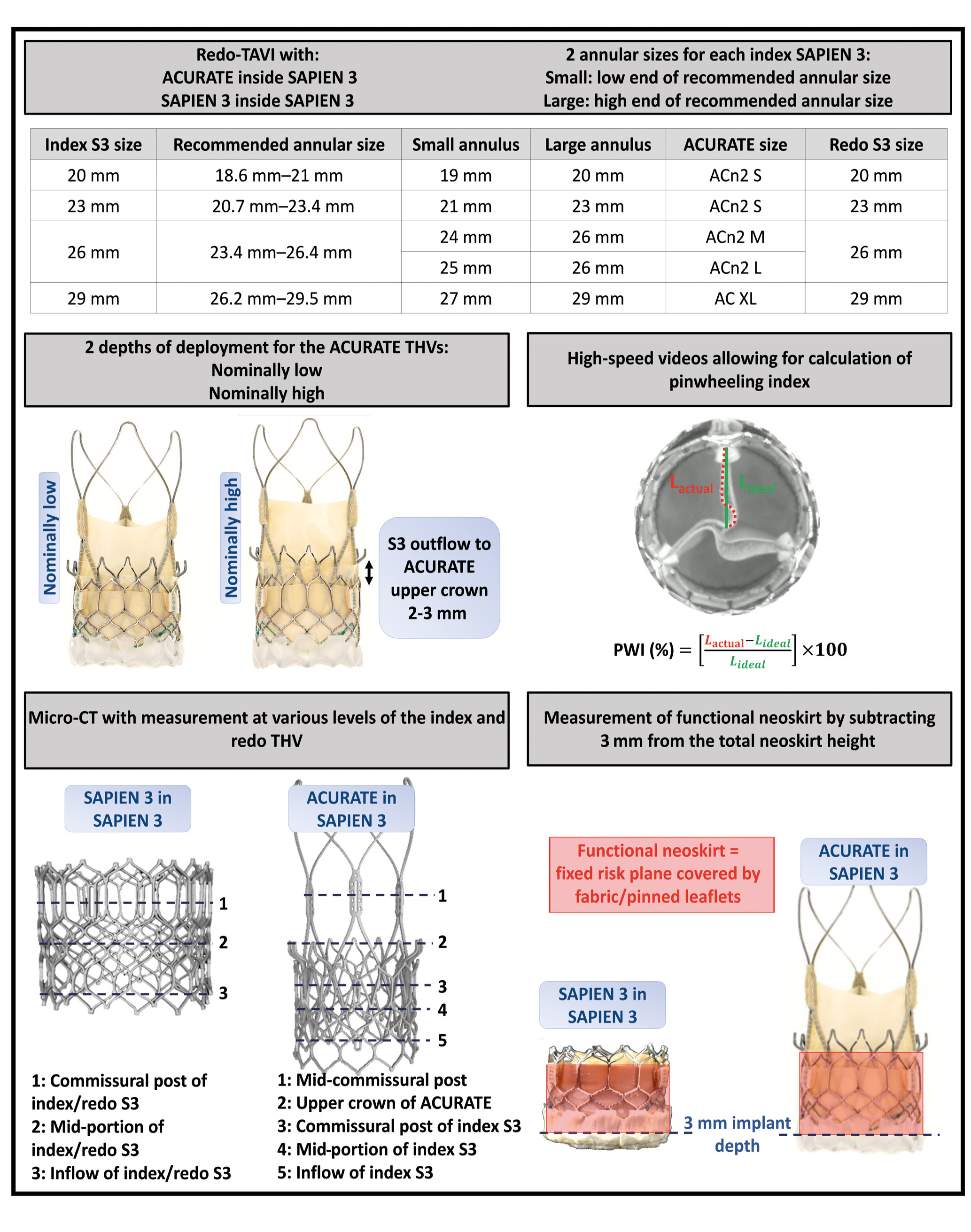
Figure 1. Study methodology. ACn2 S: ACURATE neo2 small; ACn2 M: ACURATE neo2 medium; ACn2 L: ACURATE neo2 large; AC XL: ACURATE Prime XL; CT: computed tomography; L: length; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve ; PWI: pinwheeling index
VALVES
The S3 was used as the index “failed” THV, and 20 mm, 23 mm, 26 mm and 29 mm S3 sizes were included in the study.
Two versions of the ACURATE THV were used as the redo THV: first, the ACn2 in three sizes (small [S], medium [M], and large [L]) and second, the AC XL. The AC XL valve is the next-generation, larger valve size, allowing treatment of annuli 29 mm in diameter (91 mm in perimeter), and it has improved radial strength due to a modification in the THV frame (Figure 2).
The heights of the stent body including the outer paravalvular leak (PVL) sealing skirt are 14 mm, 15 mm, 16 mm and 17 mm for the S, M, L and XL ACURATE THVs, respectively. The diameters at the commissural posts are 23 mm, 25 mm, 27 mm and 29 mm for the S, M, L and XL THVs, respectively. The diameters at the upper crown are obtained by adding 5 mm to the commissural post diameters (28 mm, 30 mm, 32 mm, and 34 mm for the S, M, L and XL, respectively) and the diameters at the lower crown by adding 3 mm to the commissural post diameters (26 mm, 28 mm, 30 mm and 32 mm for the S, M, L and XL, respectively). Of note, expansion of the upper crown of the ACURATE valves ensures nominal opening of the THV leaflets. Other characteristics of the 2 THVs have been described in detail previously6. The S3 was also used as the redo THV in order to allow comparison with the ACn2, using sizes 20 mm, 23 mm, 26 mm and 29 mm.
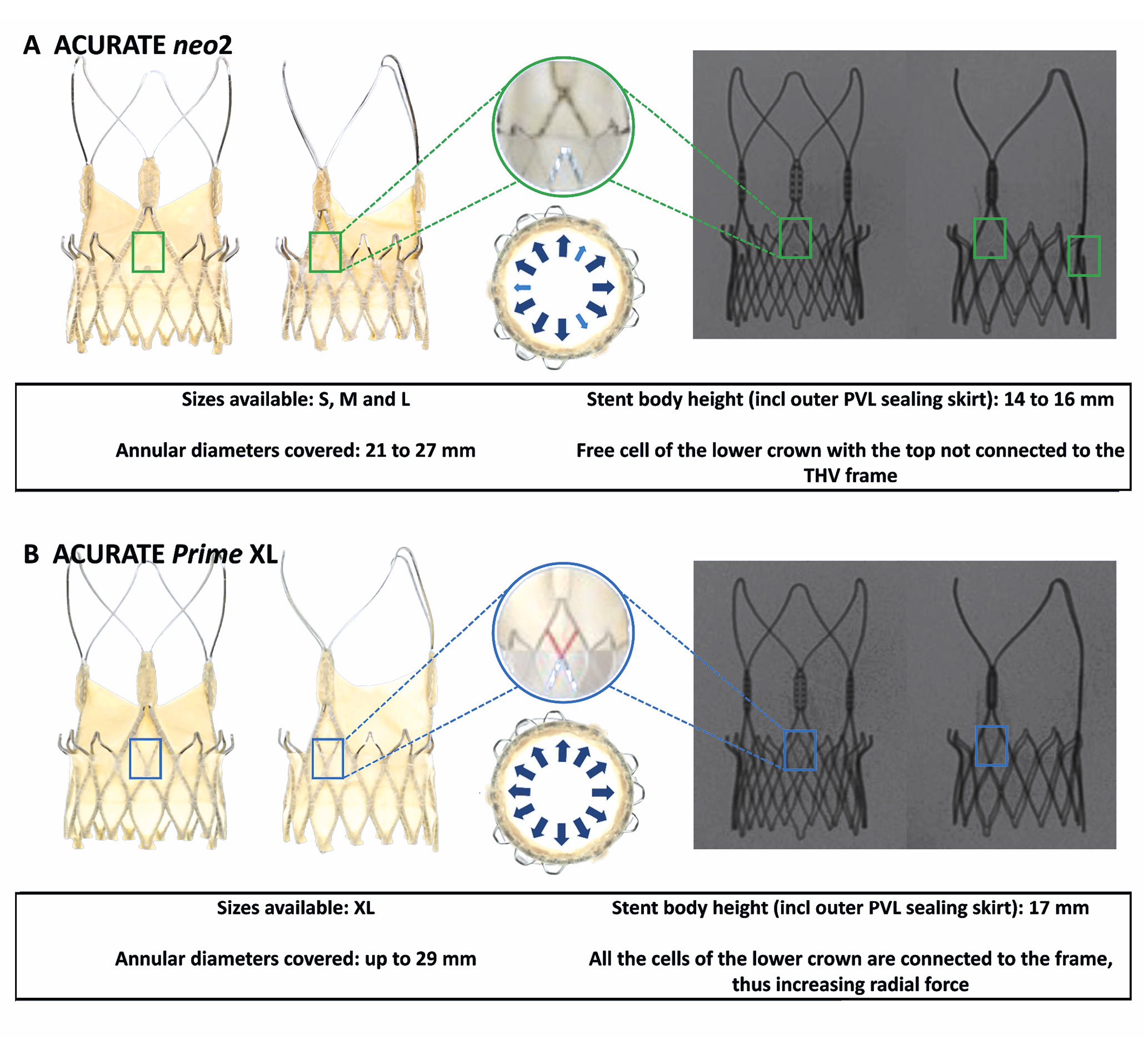
Figure 2. Features of the ACURATE neo2 and ACURATE Prime XL devices. A) ACURATE neo2; B) ACURATE Prime XL. incl: including; M: medium; L: large; PVL: paravalvular leak; S: small; THV: transcatheter heart valve
ANNULAR SIZING
The S3 manufacturer’s recommendation states that each size of valve can be implanted for a range of annular diameters/areas. Thus, the 20 mm, 23 mm, 26 mm, and 29 mm S3 can be used for the following annular diameter ranges, respectively: 18.6-21.0 mm, 20.7-23.4 mm, 23.4-26.4 mm, and 26.2-29.5 mm. To account for this range of possibilities, and since the size of native annuli will affect the expansion of the index and redo THVs, different combinations of “failed” S3 and ACURATE or redo S3 in S3 valves were deployed; these configurations are detailed in Figure 1. This allowed for an assessment of the annular range for each S3 size and the potential implications on expansion and performance following redo-TAVI.
REDO-TAVI CONFIGURATIONS
Redo-TAVI configurations were performed using a small ACn2 within a 20 mm S3, a small ACn2 within a 23 mm S3 (the small ACn2 covers annuli up to 23 mm in diameter), a medium ACn2 within a 26 mm S3, a large ACn2 within a 26 mm S3 (based on the annular range covered, both the medium and large ACn2 could potentially be suitable for a 26 mm S3), and an AC XL within a 29 mm S3 (the AC XL covers annuli up to 29 mm in diameter).
The index S3 valves, which were considered the “failed valves”, were implanted at the recommended implant depth of 3 mm below the annulus level and were deployed with nominal volume, as per the manufacturer’s recommendations. The ACn2 valves were then positioned at 2 different depths: nominally low, with the ACn2 upper crown just above the S3 outflow, and nominally high with the ACn2 upper crown 2-3 mm above the S3 outflow (Figure 1). No configuration with the upper crown positioned below the outflow of the index valve was used, as this has been shown to be associated with worse hydrodynamic performance7.
Since the “failed” S3 THVs were new valves, no predilatation was performed, but as often needed clinically, the ACn2 was postdilatated to optimise expansion of the upper crown. For post-dilatation, a non-compliant TRUE balloon (Bard Peripheral Vascular) was used. TRUE balloon sizing was based on the diameter of the annulus. Thus, the sizes used were as follows:
− 18 mm and a 20 mm for the small ACn2 in a 20 mm S3 within a 19 mm and a 20 mm annulus, respectively
− 20 mm and 22 mm for the small ACn2 in a 23 mm S3 within a 21 mm and a 23 mm annulus, respectively
− 22 mm and 24 mm for the medium ACn2 in a 26 mm S3 within a 24 mm and a 26 mm annulus, respectively
− 24 mm and 26 mm for the large ACn2 in a 26 mm S3 within a 25 mm and a 26 mm annulus, respectively
− 26 mm and 28 mm for the AC XL in a 29 mm S3 within a 27 mm and a 29 mm annulus, respectively.
As a comparator, redo-TAVI was performed using an S3 as the redo THV. For redo-TAVI in an S3, a redo THV of the same size as the index THV was selected and deployed at nominal volume while aiming for top-of-the-frame alignment and without aiming for commissural alignment. One sample was used for each combination.
HYDRODYNAMIC ASSESSMENT
A pulse duplicator test system (ViVitro Labs) was used to assess hydrodynamic performance for all redo-TAVI configurations. The index S3 THVs were placed in a silicone holder in accordance with International Organization for Standardization (ISO) 5840-3:20218. The test medium used for this study was 40% glycerol containing saline solution, with a target viscosity of 3.8 centipoise to approximate that of blood. Results were taken for 10 consecutive cycles and then averaged. High-speed video filming assessed valve kinematics from the valve outflow and inflow. Pulsatile forward flow performance was measured at a nominal beat rate of 70±1 beats/min, systolic duration of 35±5%, mean aortic pressure of 100±2 mmHg, and simulated cardiac output of 5.0±0.1 L/min. The mean gradient (mmHg), regurgitant fraction (%), and effective orifice area (cm2) were assessed. The maximum allowable regurgitant fraction in accordance with ISO 5840-3:2021 is <20%8. The total regurgitant fraction, which included closing and intervalvular regurgitation post-S3 closure, was assessed. The effective orifice area was computed according to a simplified version of the Bernoulli equation, as previously described in ISO 58408.
IMAGING PROTOCOL
Multimodality imaging was performed, including fluoroscopy using the OEC 9900 Elite Mobile C-arm X-ray system (GE HealthCare), micro-CT and high-resolution photography, for each THV, and each implantation was configured with a digital microscope (Keyence). Micro-CT was performed using a continuous cone beam quick scan to reduce dehydration of the valve tissue, which included 1,200 projections with a magnification of 3.98, focus detector distance of 536.365 mm and focus object distance of 135.000 mm with a voltage of 110 kV and a current of 200.0 μA. The frame rate detector exposure was 5 Hz with an exposure time of 200.000 ms. 3mensio Structural Heart (Pie Medical Imaging) was used to analyse, segment and measure the CT-reconstructed data of each redo-TAVI configuration. High-resolution photography was captured at a prespecified magnification and fixed camera height. High-speed video from hydrodynamic testing was also captured.
INDEX AND REDO THV FRAME EXPANSION ASSESSMENT
Following redo-TAVI, measurements were made at several levels of the index and redo THVs (Figure 1) using micro-CT images. For the ACn2, diameters were measured at the commissural posts and upper crown. For the S3, the diameters at the inflow, mid-portion and commissural posts of the frame were measured for both the index S3 and redo S3, when the latter was used as the redo THV.
PINWHEELING INDEX
Pinwheeling, as defined by the ISO guideline for THV testing, refers to the twisting of the free edges of the leaflets, resulting from excessive leaflet redundancy. A pinwheeling index (PWI) was calculated as previously reported9, using images from the high-speed videos. The PWI of each leaflet’s free edge was measured, and the mean PWI (%) of the valve was obtained from the average (Figure 1).
NEOSKIRT AND FUNCTIONAL NEOSKIRT
The neoskirt is usually defined by the height of the pinned leaflet of the first THV. In the present study, the portion of the covered frame of the ACn2 or AC XL extending above the index S3 was also included in the neoskirt measurement, as this part poses a potential risk for coronary obstruction.
For the S3 in S3 combinations, the neoskirt was defined at the top of the pinned leaflets which are at the top of the commissural tabs. The functional neoskirt is the part of the neoskirt that extends above the annular plane and is thus influenced by implant depth. Since the S3 was implanted at a depth of 3 mm, as per the manufacturer’s recommendation, the functional neoskirt height was measured for each configuration by subtracting 3 mm from the total neoskirt height (Figure 1).
DATA ANALYSIS
Hydrodynamic variables, effective orifice areas, pressure gradients, and total regurgitation fraction are reported as mean±standard deviation (SD).
Results
HYDRODYNAMIC ASSESSMENT
The hydrodynamic function of each of the combinations is detailed in Table 1 and Figure 3. Overall, most of the samples resulted in acceptable performance according to ISO standards. However, in the 20 mm S3, the use of a 20 mm S3 as a redo THV resulted in a mean gradient (MG) >20 mmHg, while the use of an ACn2 resulted in an MG <20 mmHg. The difference between the ACn2 and the S3 was less marked for the larger S3 THV sizes (Central illustration). The hydrodynamic function was only marginally affected by the size of the native annulus within a determined THV size without major differences between the S3 in S3 and ACn2 in S3 configurations. The largest difference was noted for redo-TAVI within the 23 mm S3. In this configuration, for the S3 in S3, the MG was 16.6 mmHg in the small annulus but 12.4 mmHg in the large annulus. For the small ACn2, the MG was 14.3 mmHg in the small annulus and 12.8 mmHg in the large annulus. Of note, the extent of the effect of the implantation depth of the ACn2 (nominally high vs nominally low) on hydrodynamic function was limited. Indeed, the MG was 1-2 mmHg lower at the high position for the small ACn2, and this difference was <1 mmHg for the medium and XL sizes. Finally, the use of a large ACn2 offered little advantage compared to the medium ACn2 when implanted in a 26 mm S3.
Table 1. Hydrodynamic function of the combinations tested.
| S3 and ACn2 size | Configuration | Post-dilatation TRUE balloon size | Position | Annulus diameter | EOA, cm2 | MG, mmHg | RF, % |
|---|---|---|---|---|---|---|---|
| ACn2 small20 mm S3 | 20 mm S3 in 20 mm S3 | -- | -- | 20 mm | 1.5 | 24.7 | 13.4 |
| ACn2 small in 20 mm S3 | 20 mm | Low | 1.5 | 18.8 | 4.9 | ||
| High | 1.7 | 16.3 | 7.4 | ||||
| 20 mm S3 in 20 mm S3 | -- | -- | 19 mm | 1.5 | 24.6 | 12.1 | |
| ACn2 small in 20 mm S3 | 18 mm | Low | 1.5 | 19.3 | 4.2 | ||
| High | 1.6 | 18.0 | 6.1 | ||||
| ACn2 small23 mm S3 | 23 mm S3 in 23 mm S3 | -- | -- | 23 mm | 2.2 | 12.4 | 14.7 |
| ACn2 small in 23 mm S3 | 22 mm | Low | 2.0 | 13.2 | 6.0 | ||
| High | 2.0 | 12.8 | 7.8 | ||||
| 23 mm S3 in 23 mm S3 | -- | -- | 21 mm | 1.8 | 16.6 | 12.3 | |
| ACn2 small in 23 mm S3 | 20 mm | Low | 1.7 | 15.9 | 4.9 | ||
| High | 1.8 | 14.3 | 6.3 | ||||
| ACn2 medium26 mm S3 | 26 mm S3 in 26 mm S3 | -- | -- | 26 mm | 2.6 | 9.2 | 17.0 |
| ACn2 medium in 26 mm S3 | 24 mm | Low | 2.4 | 8.7 | 6.6 | ||
| High | 2.5 | 8.1 | 7.2 | ||||
| 26 mm S3 in 26 mm S3 | -- | -- | 24 mm | 2.3 | 10.2 | 10.2 | |
| ACn2 medium in 26 mm S3 | 22 mm | Low | 2.3 | 9.7 | 3.6 | ||
| High | 2.3 | 9.2 | 5.1 | ||||
| ACn2 large26 mm S3 | ACn2 large in 26 mm S3 | 26 mm | Low | 26 mm | 2.5 | 9.4 | 11.7 |
| High | 2.5 | 8.7 | 13.1 | ||||
| ACn2 large in 26 mm S3 | 24 mm | Low | 25 mm | 2.5 | 8.7 | 10.1 | |
| High | 2.6 | 8.1 | 11.0 | ||||
| AC XL29 mm S3 | 29 mm S3 in 29 mm S3 | -- | -- | 29 mm | 2.8 | 9.2 | 23.4 |
| AC XL in 29 mm S3 | 28 mm | Low | 2.7 | 7.6 | 12.5 | ||
| High | 2.7 | 7.5 | 11.1 | ||||
| 29 mm S3 in 29 mm S3 | -- | -- | 27 mm | 2.7 | 9.1 | 20.0 | |
| AC XL in 29 mm S3 | 26 mm | Low | 2.6 | 8.0 | 9.6 | ||
| High | 2.7 | 7.5 | 9.7 | ||||
| ACn2: ACURATE neo2; AC XL: ACURATE Prime XL; EOA: effective orifice area; MG: mean gradient; RF: regurgitant fraction; S3: SAPIEN 3 | |||||||
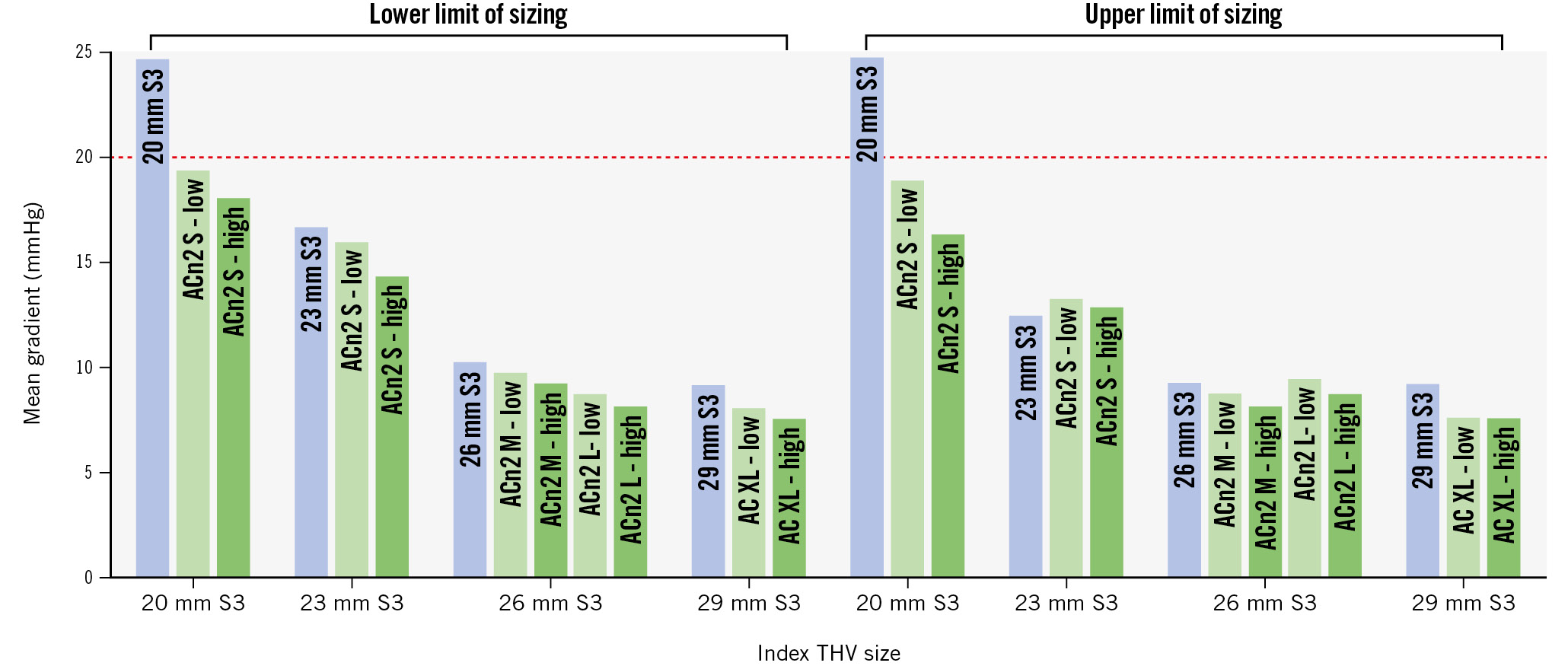
Figure 3. Graphical representation of mean gradient for each combination tested. ACn2 S: ACURATE neo2 small; ACn2 M: ACURATE neo2 medium; ACn2 L: ACURATE neo2 large; AC XL: ACURATE Prime XL; S3: SAPIEN 3; THV: transcatheter heart valve
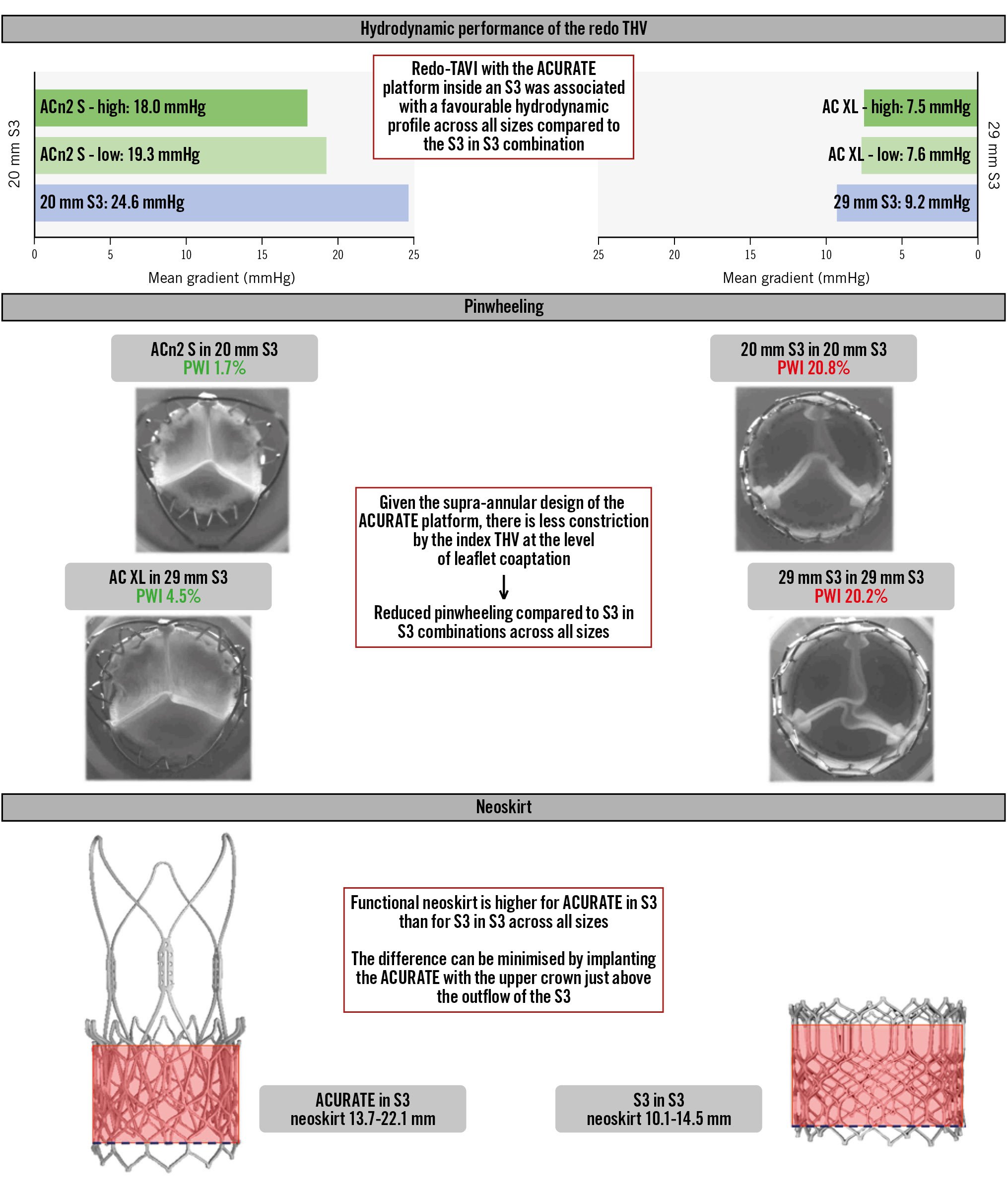
Central illustration. Redo-TAVI with the ACURATE platform for balloon-expandable transcatheter heart valve failure. ACn2 S: ACURATE neo2 small; AC XL: ACURATE Prime XL; PWI: pinwheeling index; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
REDO THV EXPANSION
As expected, all the configurations had some degree of underexpansion (Table 2). For the S3, expansion was generally quite homogeneous along the THV height, ranging between 85% and 93% of the nominal diameter. On the other hand, the ACn2 had limited constriction at the level of the mid-commissural posts, where leaflet coaptation occurs, and at the level of the upper crown, situated above the S3 outflow (overall expansion >90% of the nominal diameter). Here again, the native annulus size had limited effect on the expansion of the redo THV. However, the nominally high position allowed an almost nominal expansion at the level of the commissural posts of the ACn2. It must also be noted that the upper crown of the ACn2 extended beyond the index S3 in all the samples with a diameter exceeding the S3 diameter; for the AC XL implanted high in the 29 mm S3, this was by as much as 3.9 mm.
Table 2. Expansion of the redo THV for the combinations tested.
| S3 and ACn2 size | Configuration | Post-dilatation TRUE balloon size | Position | Annulus diameter | ACn2 measurements, mm | S3 measurements,mm | |||
|---|---|---|---|---|---|---|---|---|---|
| Comm. posts | Upper crown | Comm. posts | Mid-portion | Inflow | |||||
| ACn2 small20 mm S3 | 20 mm S3 in 20 mm S3 |
-- | -- | 20 mm | -- | -- | 17.4 | 17.4 | 17.6 |
| ACn2 small in 20 mm S3 |
20 mm | Low | 21.0 | 23.6 | -- | -- | -- | ||
| High | 22.4 | 25.6 | -- | -- | -- | ||||
| 20 mm S3 in 20 mm S3 |
-- | -- | 19 mm | -- | -- | 18.2 | 18.3 | 18.4 | |
| ACn2 small in 20 mm S3 |
18 mm | Low | 20.9 | 23.7 | -- | -- | -- | ||
| High | 21.3 | 24.6 | -- | -- | -- | ||||
| ACn2 small23 mm S3 | 23 mm S3 in 23 mm S3 |
-- | -- | 23 mm | -- | -- | 21.0 | 20.8 | 21.0 |
| ACn2 small in 23 mm S3 |
22 mm | Low | 21.5 | 24.8 | -- | -- | -- | ||
| High | 22.8 | 27.1 | -- | -- | -- | ||||
| 23 mm S3 in 23 mm S3 |
-- | -- | 21 mm | -- | -- | 19.7 | 19.6 | 19.6 | |
| ACn2 small in 23 mm S3 |
20 mm | Low | 22.0 | 25.4 | -- | -- | -- | ||
| High | 22.9 | 26.9 | -- | -- | -- | ||||
| ACn2 medium26 mm S3 | 26 mm S3 in 26 mm S3 |
-- | -- | 26 mm | -- | -- | 24.1 | 24.1 | 24.2 |
| ACn2 medium in 26 mm S3 |
24 mm | Low | 24.2 | 28.0 | -- | -- | -- | ||
| High | 24.5 | 28.8 | -- | -- | -- | ||||
| 26 mm S3 in 26 mm S3 |
-- | -- | 24 mm | -- | -- | 23.8 | 23.4 | 23.6 | |
| ACn2 medium in 26 mm S3 |
22 mm | Low | 23.7 | 27.3 | -- | -- | -- | ||
| High | 24.3 | 28.4 | -- | -- | -- | ||||
| ACn2 large26 mm S3 | ACn2 large in 26 mm S3 |
26 mm | Low | 26 mm | 25.3 | 28.5 | -- | -- | -- |
| High | 25.5 | 29.6 | -- | -- | -- | ||||
| ACn2 large in 26 mm S3 |
24 mm | Low | 25 mm | 25.2 | 28.2 | -- | -- | -- | |
| High | 26.0 | 29.4 | -- | -- | -- | ||||
| AC XL29 mm S3 | 29 mm S3 in 29 mm S3 |
-- | -- | 29 mm | -- | -- | 26.4 | 26.2 | 26.2 |
| AC XL in 29 mm S3 |
28 mm | Low | 27.9 | 32.5 | -- | -- | -- | ||
| High | 28.6 | 32.8 | -- | -- | -- | ||||
| 29 mm S3 in 29 mm S3 |
-- | -- | 27 mm | -- | -- | 27.1 | 26.5 | 26.7 | |
| AC XL in 29 mm S3 |
26 mm | Low | 27.7 | 30.9 | -- | -- | -- | ||
| High | 28.0 | 32.5 | -- | -- | -- | ||||
| ACn2: ACURATE neo2; AC XL: ACURATE Prime XL; Comm.: commissural; S3: SAPIEN 3; THV: transcatheter heart valve | |||||||||
INDEX THV EXPANSION
Expansion of the index THVs can be found in Supplementary Table 1. Overall, a small annulus was associated with a slightly smaller diameter of the index THV for all combinations. Importantly, no configuration led to notable overexpansion of the index THV beyond its nominal size.
PINWHEELING
The S3 in S3 redo-TAVI combinations had worse pinwheeling compared to the ACn2 configurations. All the S3 in S3 combinations had a PWI >10% (up to 20.8% for the 20 mm S3 in 20 mm S3 combination), while most of the ACn2 in S3 combinations had a PWI <5%, with the PWI in small annuli below 2%. Only the ACn2 L in the 26 mm S3 had a PWI above 5%, but still below 10%. Additionally, pinwheeling persisted across all S3 sizes and was not limited to small THVs. For the S3 in S3, the smaller annulus was associated with increased pinwheeling, but there was little difference for the ACn2. The nominally high implantation was not associated with notably lower pinwheeling than the nominally low implantation for the ACn2. Detailed pinwheeling assessment can be found in Table 3 and Figure 4.
Table 3. Pinwheeling index for the combinations tested.
| S3 and ACn2 size | Configuration | Post-dilatation TRUE balloon size | Position | Annulus diameter | Mean pinwheeling index, % |
|---|---|---|---|---|---|
| ACn2 small20 mm S3 | 20 mm S3 in 20 mm S3 | -- | -- | 20 mm | 17.1 |
| ACn2 small in 20 mm S3 | 20 mm | Low | 1.7 | ||
| High | 0.4 | ||||
| 20 mm S3 in 20 mm S3 | -- | -- | 19 mm | 20.8 | |
| ACn2 small in 20 mm S3 | 18 mm | Low | 1.7 | ||
| High | 0.5 | ||||
| ACn2 small23 mm S3 | 23 mm S3 in 23 mm S3 | -- | -- | 23 mm | 11.8 |
| ACn2 small in 23 mm S3 | 22 mm | Low | 0.9 | ||
| High | 0.2 | ||||
| 23 mm S3 in 23 mm S3 | -- | -- | 21 mm | 11.7 | |
| ACn2 small in 23 mm S3 | 20 mm | Low | 1.2 | ||
| High | 1.2 | ||||
| ACn2 medium26 mm S3 | 26 mm S3 in 26 mm S3 | -- | -- | 26 mm | 12.4 |
| ACn2 medium in 26 mm S3 | 24 mm | Low | 0.6 | ||
| High | 0.0 | ||||
| 26 mm S3 in 26 mm S3 | -- | -- | 24 mm | 17.0 | |
| ACn2 medium in 26 mm S3 | 22 mm | Low | 1.6 | ||
| High | 1.9 | ||||
| ACn2 large26 mm S3 | ACn2 large in 26 mm S3 | 26 mm | Low | 26 mm | 6.8 |
| High | 1.3 | ||||
| ACn2 large in 26 mm S3 | 24 mm | Low | 25 mm | 5.5 | |
| High | 6.8 | ||||
| AC XL29 mm S3 | 29 mm S3 in 29 mm S3 | -- | -- | 29 mm | 12.9 |
| AC XL in 29 mm S3 | 28 mm | Low | 4.8 | ||
| High | 4.5 | ||||
| 29 mm S3 in 29 mm S3 | -- | -- | 27 mm | 20.2 | |
| AC XL in 29 mm S3 | 26 mm | Low | 4.5 | ||
| High | 3.8 | ||||
| ACn2: ACURATE neo2; AC XL: ACURATE Prime XL; S3: SAPIEN 3 | |||||
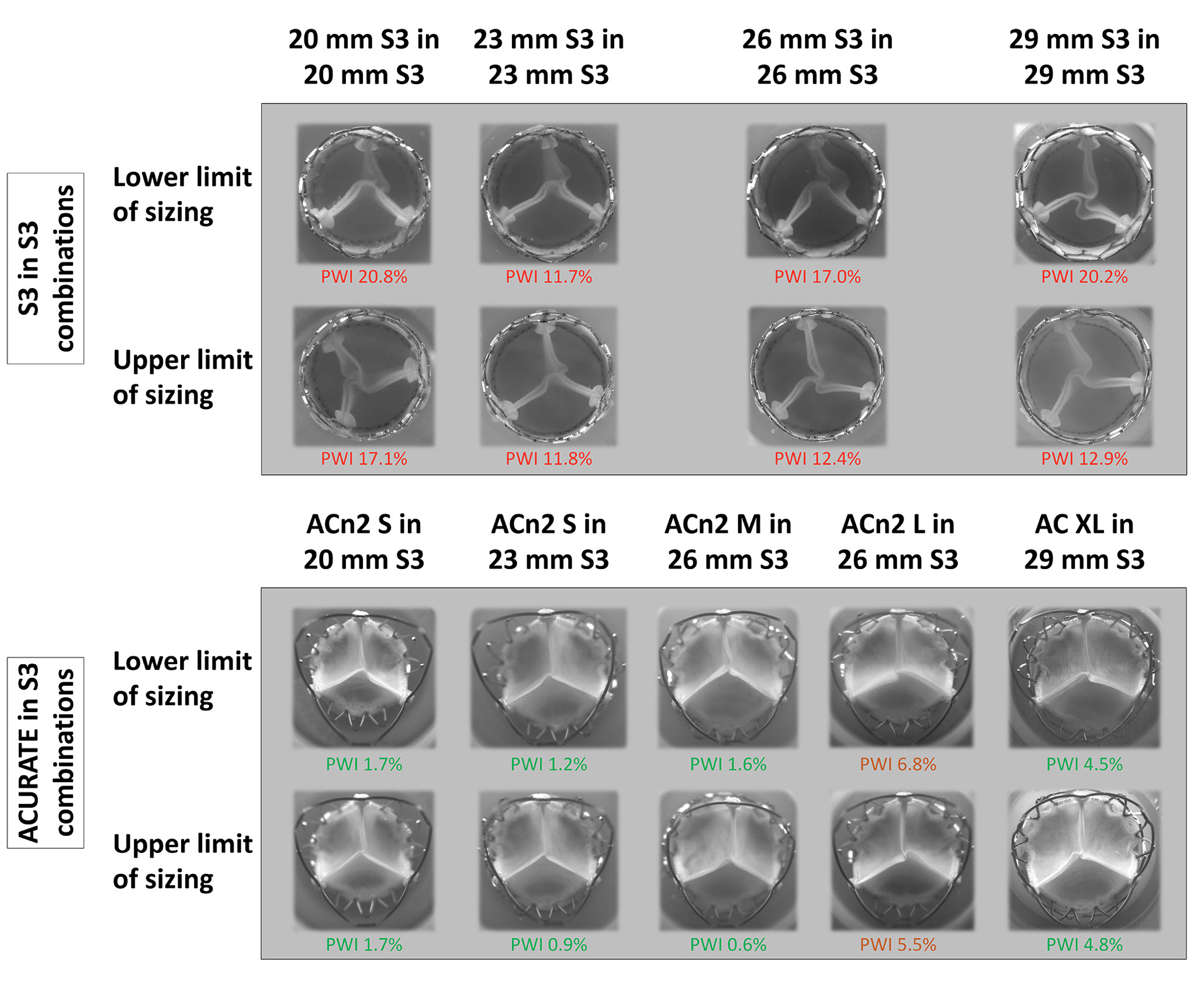
Figure 4. Images from the high-speed videos showing the degree of pinwheeling for each combination. For the ACURATE in S3, only combinations with a nominally low implantation are shown. ACn2 S: ACURATE neo2 small; ACn2 M: ACURATE neo2 medium; ACn2 L: ACURATE neo2 large; AC XL: ACURATE Prime XL; PWI: pinwheeling index; S3: SAPIEN 3
FUNCTIONAL NEOSKIRT
The ACn2 implanted within the S3 resulted in functional neoskirt heights ranging between 13.7 mm and 22.1 mm, depending on the implant depth and THV size (Figure 5). The functional neoskirt heights were shortest in the low implantation position, with the lowest value (13.7 mm) for the ACn2 S implanted in a nominally low position in the 20 mm S3. A higher functional neoskirt height was noted in the high implantation position, with the highest value (22.1 mm) obtained for the AC XL implanted in the 29 mm S3. As a comparison, the S3 in S3 combination resulted in lower functional neoskirt heights, ranging from 10.1 mm to 14.5 mm when considering the top of the commissural tabs and 12.9 mm to 19.8 mm when considering the top of the frame.
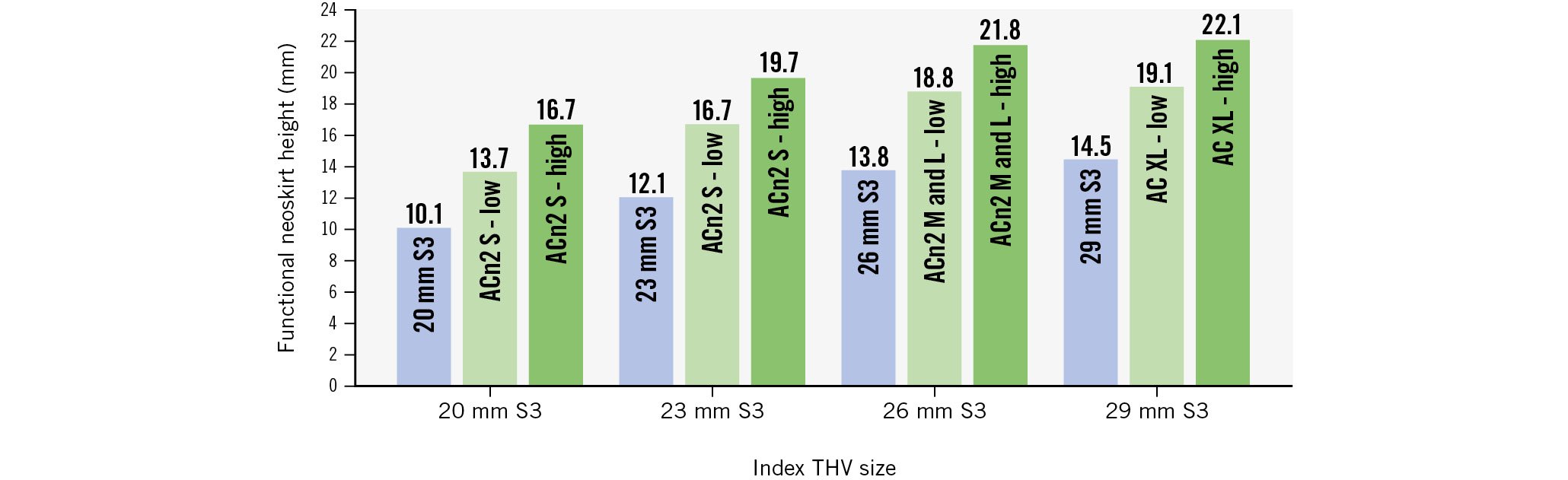
Figure 5. Graphical illustration of functional neoskirt height according to THV type and size. ACn2 S: ACURATE neo2 small; ACn2 M: ACURATE neo2 medium; ACn2 L: ACURATE neo2 large; AC XL: ACURATE Prime XL; S3: SAPIEN 3; THV: transcatheter heart valve
Discussion
The current study shows that 1) redo-TAVI with the ACn2 or the new AC XL in S3 was associated with a more favourable hydrodynamic performance across all THV sizes compared to the S3 in S3 combination. 2) The supra-annular design of the ACn2/AC XL makes them less susceptible to constriction by the index THV at the level of leaflet coaptation, with consequently less pinwheeling. 3) The diameter of the ACn2/AC XL upper crown can exceed the diameter of the index S3 by almost 4 mm. 4) A nominally high implantation of the ACn2/AC XL offers minimal advantage compared to a nominally low implantation. 5) When the native annular diameter is relatively small compared to the index THV, the ACn2/AC XL is less affected than the S3 when used as the redo THV. 6) Redo-TAVI with the ACn2/AC XL inside an S3 resulted in a slightly taller neoskirt compared to the S3 in S3 combination.
Redo-TAVI represents a clinical challenge, since there is a need to avoid coronary obstruction and to preserve coronary access while optimising THV performance. Short-frame THVs offer the advantage of a shorter neoskirt but may be associated with higher residual gradients, while tall-frame THVs create a taller neoskirt and might compromise coronary access41011. The findings of the present study suggest that the ACn2/AC XL platform might offer a compromise through its unique design for redo-TAVI. Indeed, when used as the redo THV, the ACn2/AC XL consistently resulted in an acceptable gradient, with a particular advantage for smaller valve sizes compared to the S3 in S3 combinations. In addition, all ACn2/AC XL combinations had very limited pinwheeling compared to the S3 in S3 combinations. This latter finding is explained by the fact that the function of the ACn2/AC XL platform depends on proper expansion of the upper crown and commissural post but is less influenced by the lower crown, which is more susceptible to being constrained in an underexpanded index THV. By contrast, the S3 – which is implanted at the same depth as the index THV – requires optimal expansion at every level, which can be challenging to achieve within a degenerated and potentially underexpanded index THV. The clinical implications of non-homogeneous expansion and pinwheeling remain controversial, but they have been linked to increased risk of thrombosis and potentially reduced durability1213.
Despite the taller neoskirt observed with the ACn2/AC XL compared to the S3 in S3 combination, it is likely that the ACURATE platform offers the best chance of preserving coronary access when using a tall-frame THV, as suggested by prior clinical and bench data14151617. Additionally, it appears that neoskirt height can be minimised by using the low implant with the upper crown just above the outflow of the S3, since a higher implant did not seem to offer much gain in terms of hydrodynamic function or pinwheeling and may potentially impact coronary access.
Finally, the present study offers some insights for risk mitigation strategies in the context of redo-TAVI. Indeed, we show that the upper crown of the ACn2/AC XL THVs extends beyond the index THV. This could potentially have significant implications in terms of the risk of aortic root injury, especially when performing aggressive post-dilatation where the upper crown could come into contact with the aortic wall. This seems particularly true for the combinations inside the 20 mm S3 – which are usually implanted in patients with a small aortic root – where the upper crown diameter was >5 mm larger than the S3.
Limitations
Bench testing design may not reflect physiological conditions in clinical practice. Indeed, the index valves were new, and hence in a degenerated in vivo failed valve which is calcified, expansion of the index THV may vary compared to a bench model. Additionally, it is possible that combining pre- and post-dilatation in the index S3 might have led to improved performances of the S3 in S3 combination18. Moreover, in the context of a degenerated and/or constricted S3, an undersizing strategy, in which a smaller S3 is selected in order to allow for more complete expansion and potentially reduced pinwheeling, still needs to be investigated. It must also be noted that the S3 and not the SAPIEN 3 Ultra (Edwards Lifesciences) was used in this study and that it is unclear if the enhanced outer skirt of the Ultra version would lead to even more underexpansion of the valve when used as the redo THV. Finally, due to the difficulty of obtaining THVs for the purpose of bench testing, only one sample per combination was available.
Conclusions
Redo-TAVI with an ACn2/AC XL within an index S3 led to favourable THV haemodynamics and minimal leaflet pinwheeling compared to an S3 in S3 combination, particularly in cases with a small annulus compared to the index THV size. The S3 in S3 combinations, however, had a lower neoskirt.
Impact on daily practice
The ACURATE platform has been associated with favourable outcomes in TAVI. Its potential role for the treatment of balloon-expandable valve failure remains unknown. This study suggests that the ACURATE platform is associated with a favourable hydrodynamic outcome, particularly in the smaller valve sizes, and minimal pinwheeling, across all valve sizes, compared to the S3 in S3 combinations. Clinical series are required to further understand the procedural challenges and clinical outcomes when performing redo-TAVI using the ACURATE platform.
Conflict of interest statement
D. Grant and C. Frawley are employees of Boston Scientific. M. Akodad is a consultant for Edwards Lifesciences and Medtronic. U. Landes is a consultant for Edwards Lifesciences. M. Rinaldi receives advisory board/consultant/speaker honoraria from Boston Scientific, Edwards Lifesciences, and Abbott. A.A. Khokhar has received speaker fees from Boston Scientific. D. Dudek has received research funding from Boston Scientific. W. Kim has received personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Hi-D Imaging, Meril Life Sciences, and Shockwave Medical; and research funding from Boston Scientific. S. Yakubov receives consulting/advisory board honoraria from Medtronic and Boston Scientific. P Sorajja is a consultant to 4C Medical, Anteris, Abbott Structural, BioSense Webster, Boston Scientific, Edwards Lifesciences, Evolution Medical, Foldax, GE HealthCare, GLG, Medtronic, Philips, Siemens, Shifamed, W.L. Gore & Associates, VDyne, and xDot. G. Tarantini has received lecture fees from Edwards Lifesciences, Medtronic, Abbott, Boston Scientific, and Abiomed. J.G. Webb is a consultant to and has received research funding from Edwards Lifesciences, Abbott, and ViVitro Labs. D.A. Wood is a consultant to and has received research funding from Edwards Lifesciences and Abbott. S.L. Sellers is a consultant for Edwards Lifesciences, Anteris, Excision Medical, and Medtronic; and has received funding from Edwards Lifesciences, Medtronic, and HeartFlow Inc. J. Sathananthan is an employee of Boston Scientific; and has received research funding from Edwards Lifesciences and Medtronic. The other authors have no relevant conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

