
Abstract
Aims: Implantation of a transcatheter heart valve to treat valve failure is an attractive option which is being used more frequently. Despite large clinical experience there is a paucity of in vitro data to support this treatment. Our aim was to provide in vitro performance data on the SAPIEN XT transcatheter heart valve implanted within two types of pericardial surgical heart valve.
Methods and results: A SAPIEN XT was implanted within multiple sizes, i.e., 21, 23, 25 of Magna Ease and explanted calcified PERIMOUNT valves. Each combination underwent in vitro testing according to the ISO 5840 guidance. Combinations were evaluated for embolisation and acute and long-term haemodynamic performance. All combinations met the ISO 5840 minimum requirements for effective orifice area (EOA) and regurgitant fraction at the initiation of the study, after 20 million and 200 million cycles. EOA was above 1.4 cm2 for all valve combinations with Magna Ease and above 1.22 cm2 with PERIMOUNT combinations. Regurgitant fraction was ≤7% for SAPIEN XT-Magna Ease combinations and was ≤10% for SAPIEN XT-PERIMOUNT combinations, which also met the ISO requirements. No embolisation was observed. The largest migration was 1.2 mm.
Conclusions: In vitro tests demonstrate excellent haemodynamic performance and durability in the six VIV combinations which are commonly seen in clinical practice.
Abbreviations
AWT: accelerated wear testing
EOA: effective orifice area
ID: internal diameter
ISO: International Organization for Standardization
SHV: surgical heart valve
SVD: structural valve deterioration
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
VIV: valve-in-valve
Introduction
The numbers of bioprosthetic heart valves implanted annually in the world are on the rise1. With the lowering of the cut-off age for bioprosthetic surgical heart valve (SHV) implantation, and an ageing population, one can expect an increase in the number of SHVs experiencing structural valve deterioration (SVD)1,2. Re-do operations carry an increased risk of operative mortality. The operative risk of elective re-do aortic valve surgery has been reported to range from 2%-7%, but can be greater than 30% in high-risk, non-elective patients3.
Transcatheter heart valve (THV) implantations into degenerated SHVs have been performed by an increasing number of physicians as an alternative to re-do surgery in high-risk patients and are referred to as valve-in-valve (VIV)4-10. VIV is an attractive option as it avoids a sternotomy and cardiopulmonary bypass and can potentially accelerate patient recovery and reduce length of hospital stay11. A large body of experience exists on the use of the SAPIEN XT THV (SAPIEN XT) balloon-expandable valve (Edwards Lifesciences, Irvine, CA, USA) and self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA) to treat degenerated aortic SHVs4-11.
There are at least 16 different types of SHV which have been implanted in the last two decades12-14. Each SHV has a unique design, which can influence its compatibility with a given THV. Most users extrapolate information gained from transcatheter aortic valve implantation (TAVI) experience to perform a VIV procedure. This is especially true when choosing an appropriately sized THV for a given SHV11,12. Although there is guidance available on sizing as well as positioning of THV, limited in vitro or in vivo animal data are available regarding haemodynamic performance and durability of the THV after a VIV procedure14-16. Similarly, in vitro data on the ability of a VIV combination to resist embolisation are sparse.
Edwards Lifesciences has been a leading manufacturer of both SHVs and THVs. This has provided a unique opportunity to assess in vitro performance data of the SAPIEN XT Model 9300TFX THV deployed into a Carpentier-Edwards PERIMOUNT Magna Ease Model 3300TFX (Magna Ease; Edwards Lifesciences) and explanted Carpentier-Edwards PERIMOUNT Models 2800 and 2900 (PERIMOUNT; Edwards Lifesciences). In this paper we discuss the clinically relevant findings of these tests with a focus on the ability of various VIV combinations to resist embolisation, on post-implant gradients and on long-term haemodynamic performance.
Methods
This study was conducted to evaluate the resistance to migration and 200 million cycle durability of the SAPIEN XT when deployed in a Magna Ease and explanted calcified PERIMOUNT pericardial valves under hypertensive conditions. A nominally deployed SAPIEN XT was used as a reference article. Important design characteristics of the three valve types under consideration are as follows:
CARPENTIER-EDWARDS PERIMOUNT DESIGN (MODEL 2800 AND MODEL 2900)
PERIMOUNT is one of the earlier versions of pericardial SHV intended for an intra-annular implantation in the aortic position. The sewing ring is higher than the bottom of the basal band (Figure 1A). It is made of three components: the frame, polyester sewing ring and three matched bovine pericardial leaflets. The frame has three posts with cobalt-chromium wire and basal band (solid but with multiple suture holes), which are visible under fluoroscopy.
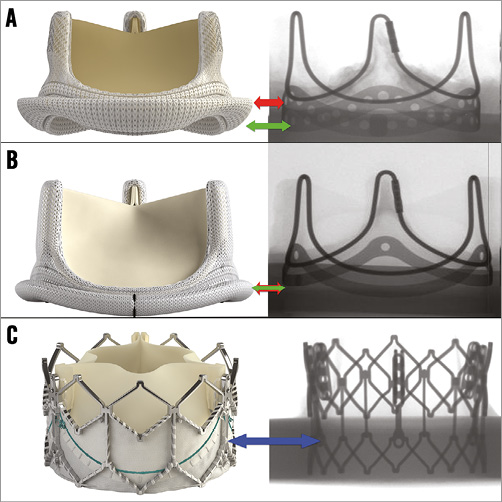
Figure 1. Various heart valves used for the study. A) PERIMOUNT SHV. Side view and fluoroscopic appearance. Green arrow points to the basal band and red arrow points to the sewing ring, i.e., intra-annular design. B) Magna Ease. Side view and fluoroscopic appearance. Green arrow points to the basal band and red arrow points to the sewing ring, i.e., supra-annular design. Both arrows are at the same level. C) SAPIEN XT. Side view and fluoroscopic view. Blue arrow points to the nadir of the pericardial leaflets.
There is no clinically significant difference between Model 2800 (intended for the US market) and Model 2900 (intended for Europe and the rest of the world).
CARPENTIER-EDWARDS PERIMOUNT MAGNA EASE DESIGN (MODEL 3300TFX)
Magna Ease is the latest generation of pericardial SHV intended for supra-annular implantation in the aortic position. The sewing ring is at the same level as the bottom of the basal band (Figure 1B). It is also made of three components: the valve frame, polyester sewing ring and three matched bovine pericardial leaflets. The valve frame has three posts with cobalt-chromium wire and basal band (solid), which are visible under fluoroscopy.
SAPIEN XT DESIGN
The SAPIEN XT (Figure 1C) is a second-generation balloon-expandable THV with a cobalt-chromium frame. Three matched bovine pericardial leaflets, similar to those of the Magna Ease pericardial valve, are sutured within the frame.
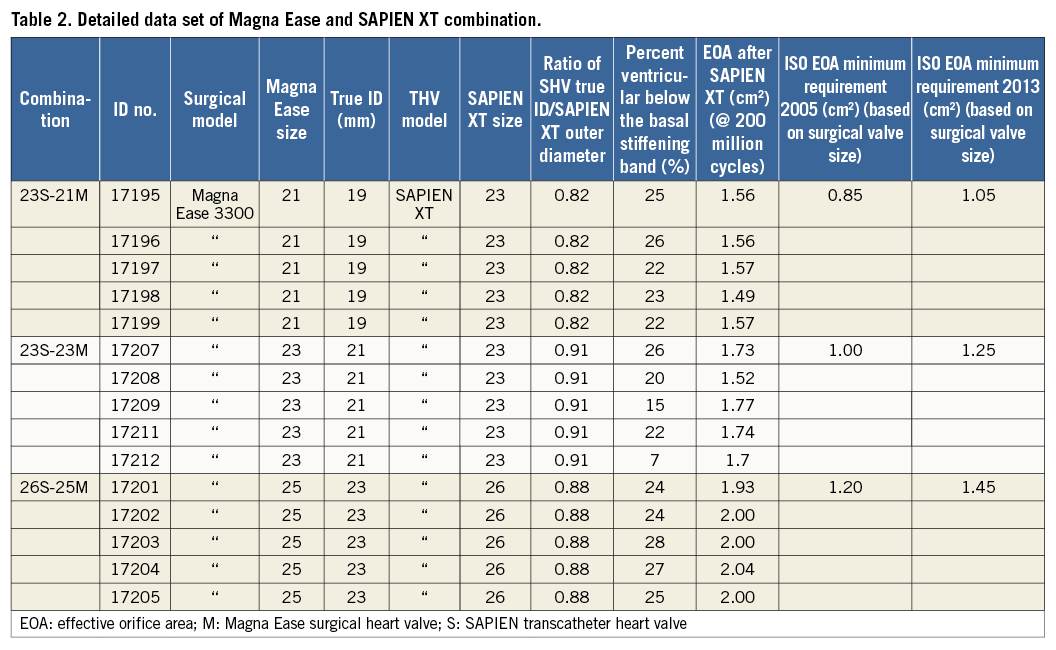
Details of the sizes and important dimensions of all three valves are provided in Table 1.
Pulse duplicator hydrodynamic testing and accelerated wear testing systems are used to generate important functional data. Testing was carried out in accordance with ISO 5840 guidance for surgical heart valves. ISO 5840:2009 is applicable to all devices intended for implantation in human hearts, as a heart valve substitute. ISO 5840 specifies in vitro and preclinical in vivo testing and clinical evaluations. This International Standard also covers important hydrodynamic and durability characteristics of heart valve substitutes and imposes minimum performance requirements for heart valve substitutes where adequate scientific and/or clinical evidence exists for their justification. In 2013, the ISO committee released ISO 5840-3:2013 which is specifically for heart valve substitutes delivered by transcatheter techniques. Both ISO 5840:2009 and 5840-3:2013 were considered in this evaluation. This is explained in brief below.
PULSE DUPLICATOR
The test and reference valves were tested using the open-loop Cardiac Valve Analyzer, pulse duplicator system (Edwards Lifesciences) (Figure 2). The pulse duplicator is used to perform hydrodynamic testing. It essentially simulates what a ventricle does and has sensors to detect pressures and flows, and has controls which allow adjustments of important parameters such as heart rate (beat rate), flow and back pressures. Thus, it provides hydrodynamic information on the fluid mechanical performance of the heart valve substitute. The pulsatile-flow testing conducted in the pulse duplicator approximates the physiological conditions of the intended device application in accordance with guidelines of the ISO 5840 standard for cardiovascular implants.
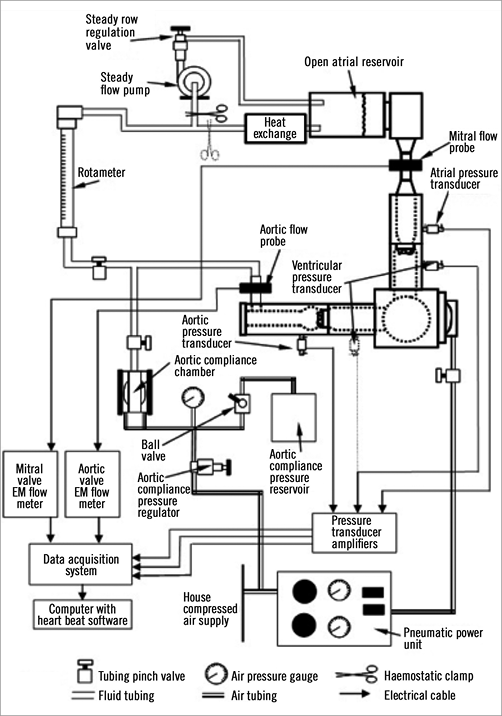
Figure 2. Schematic diagram of the pulse duplicator circuit used for haemodynamic testing.
Accelerated wear testing (AWT) system
The test and reference valves were tested in the Edwards modified Dynatek M6 Heart Valve Accelerated Wear Tester. The valves were cycled at accelerated rates for a minimum of 20 million cycles at 160 mmHg peak differential closing pressure and an additional 180 million cycles at 95 mmHg peak differential closing pressure. AWT is used to cycle the valves rapidly to test durability but does not generate hydrodynamic performance data. Hence, typically every 50 million cycles the valves are removed from the AWT machines and then put in a pulse duplicator to assess valve function such as pressure gradients and leakage. The THV was also assessed for movement within the SHV. At 75 beats per minute, there are approximately 40 million heart beats per year. Although heart beats in a patient may not be the same as heart beats in the AWT, 20 million cycles equate to approximately six patient months if the heart beat rate was 75 beats per minute. This translates to five years for 200 million cycles.
All AWT testers were tuned to achieve full valve closure and opening as compared to the same valve observed under pulsatile flow conditions in the pulse duplicator at 5 lpm, 70 bpm, 100 mmHg mean arterial pressure, mean leakage pressure of 160 mmHg (for the first 20 million cycles), and 35±2% aortic forward flow ratio. Figure 3 shows an example of the tested valve opening and closing motions observed in the AWT.
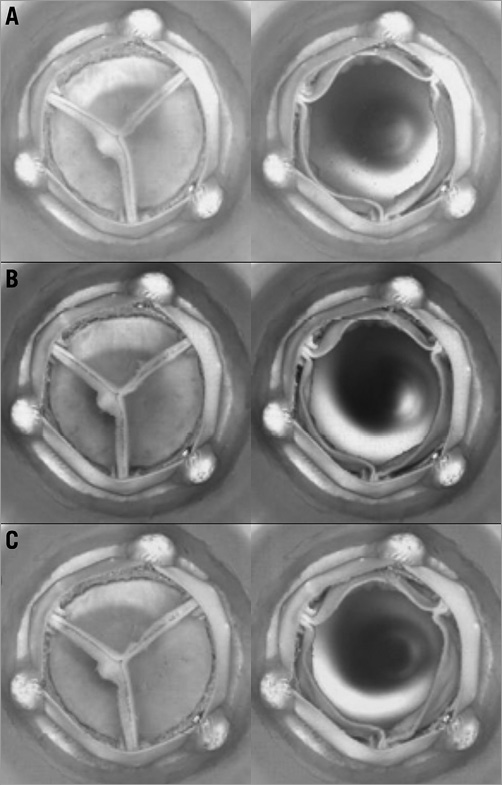
Figure 3. Examples of test sample opening and closing at the start (A), after 20 million cycles (B), and after 200 million cycles (C).
The study was conducted in two parts:
PART A: IN VITRO VIV DURABILITY STUDY OF SAPIEN XT DEPLOYED IN A MAGNA EASE
This study was conducted to evaluate the resistance to migration and 200 million cycle durability of the SAPIEN XT when deployed in a Magna Ease under hypertensive conditions. Nominally deployed SAPIEN XT were used as reference articles.
To evaluate the ability of the SAPIEN XT to resist embolisation, the valve was subjected to a number of pulsatile and steady state conditions that represent extreme dislodgement pressures for the target patient population. For this study, valve embolisation was defined as the dislodgement of the SAPIEN XT from the intended and documented original position to an unintended and non-therapeutic location as defined in ISO 5840.
The VIV configurations below were used in the testing:
– 23 mm SAPIEN XT in a 21 mm Magna Ease
– 23 mm SAPIEN XT in a 23 mm Magna Ease
– 26 mm SAPIEN XT in a 25 mm Magna Ease
– Two 23 mm and one 26 mm SAPIEN XT used as controls
Five samples of each VIV configuration were tested. Tested valves were referenced by a unique identifier (ELX number) used by Edwards Lifesciences to document the valve for in vitro testing. All test valves were crimped on NovaFlex+ delivery systems (Edwards Lifesciences) and underwent deployment through a simulated aortic arch crossing.
Valve performance testing was conducted in accordance with the requirements of the Cardiovascular Implants - Cardiac Valve Prostheses International Standard Guidance: ISO 5840:2005 (section 7.2.3 and Annex L4). The Edwards modified Dynatek M6 AWT was used to simulate hypertensive pressures of 160 mmHg for 20 million cycles (equivalent to six months) and normotensive pressures of 95 mmHg for an additional 180 million cycles at a rate range of 1,000-1,200 bpm (beats per minute). In order to assess the impact of each test condition on migration (precursor to valve embolisation), the valves were subjected to macroscopic inspection, X-ray migration measurement, and photo documentation at each test interval (e.g., pre-pulsatile, post-normotensive pulsatile, post-steady forward flow, post-steady back-pressure, post-hypertensive pulsatile, and post-20 million cycles and at 50, 100, 150, and 200 million cycles AWT). The valves were mounted as shown in Figure 4A and Figure 4B.
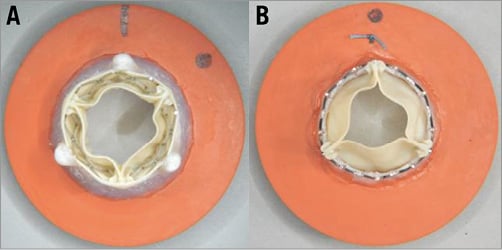
Figure 4. Valve mounting for testing. A) Outflow view of a representative sample of VIV in a valve mount. The combination shown is 23 SAPIEN XT in a 23 Magna Ease. B) Outflow view of a representative sample of a 23 SAPIEN XT mounted in a valve mount as a control.
STAGES OF VALVE PERFORMANCE TESTING
Pre-pulsatile means, shortly after the THV was placed inside the surgical valve, the inspections and X-rays were performed, before any testing of the valves.
Post-normotensive pulsatile means, after testing in a pulse duplicator (cardiac simulator or PD) under normotensive conditions in the aortic position, the inspections and X-rays were performed, before any testing of the test valves. This test lasts about 10 to 30 minutes depending on the ease of adjusting the machine.
Post-steady forward flow means, after testing in a non-pulsatile device where flow was stepped up from a low of 5 lpm (litres per minute) up to 30 lpm in 5 lpm increments, the inspections and X-rays were performed, before any testing of the test articles. This test lasts about 10 to 30 minutes depending on the ease of adjusting the machine.
Post-steady back flow means, after testing in a non-pulsatile device where the valve is closed and back pressure is steadily increased from 40 mmHg to 80, to 120, to 160 and to 200 mmHg, the inspections and X-rays were performed, before any testing of the test valves. This test lasts about 10 to 30 minutes depending on the ease of adjusting the machine.
Post-hypertensive pulsatile means, after testing in a pulse duplicator (cardiac simulator) under hypertensive conditions of 160 mmHg in the aortic position, the inspections and X-rays were performed, before any testing of the test valves. This test lasts about 10 to 30 minutes depending on the ease of adjusting the machine.
Post-20 million AWT means after cycling in a durability tester for 20 million cycles under hypertensive conditions of 160 mmHg in the aortic position. Note, only the first 20 million cycles in the pulse duplicator are run at pressures >160 mmHg. This test in the PD lasts about 10 to 30 minutes depending on the ease of adjusting the machine.
Subsequent testing to 200 million cycles means, after the 20 million cycle test at 160 mmHg and the inspections, the valves were put back in the durability testers and run to 200 million cycles (equivalent to five years of heart beats) and normotensive pressures exceeding 95 mmHg. At 50 million cycles and every 50 million cycles thereafter, the testers are stopped, the valves removed and the same inspections and tests are performed before the valves go back in the tester.
At the end of 200 million cycles, we subsequently did a few pull tests on the non-explant combinations. This was not intended originally but was performed after an FDA request. We can provide these data for you if you wish.
VALVE-IN-VALVE DEPLOYMENT
DEPTH OF IMPLANT
In this study, the outflow edge of the SAPIEN XT was aligned just below the free edge of the Magna Ease leaflets. This position was determined to be an optimal placement because there is no SHV leaflet overhang and sufficient overlap between the THV frame and SHV basal band to anchor the THV securely. In Figure 5, a representative VIV configuration shows the vertical positioning. The percent ventricular positioning ranged from 7% to 28% for the test valves (Table 2). The percent ventricular positioning varies depending on the relative heights of the SHV and THV.
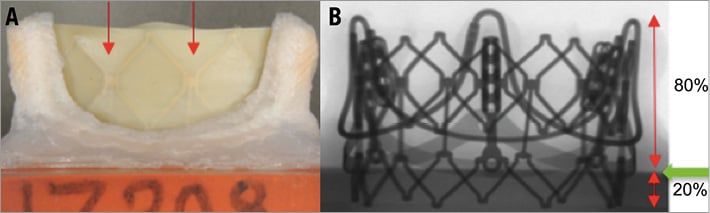
Figure 5. THV frame position within Magna Ease. A) Side view of a 23 Magna Ease with 23 SAPIEN XT frame visible through the leaflets (red arrows). Deployment was performed to align the top of the THV with the top of the leaflets of the SHV. B) Fluoroscopic view of the same combination demonstrates that 20% of the SAPIEN XT frame is below the basal band, i.e., ventricular.
ORIENTATION
The orientation (commissural angle offset) for the final deployment of the SAPIEN XT in the Magna Ease was targeted to be evenly distributed between the two extremes, 0 degrees (frame post to frame post) and 60 degrees (halfway between two frame posts), with the remaining sample oriented at 30 degrees (halfway between previous two samples) (Figure 6).
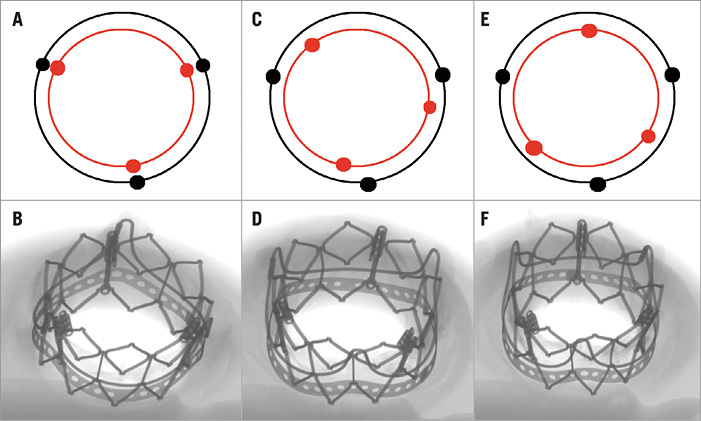
Figure 6. Orientation of the SAPIEN XT in relation to the frame posts of the SHV. A) & B) Stent post to frame post deployment – 0 degree orientation. C) & D) 30 degree orientation. E) & F) 60 degree orientation.
PART B: IN VITRO VIV DURABILITY STUDY OF SAPIEN XT DEPLOYED INTO EXPLANTED CALCIFIED PERIMOUNT VALVES
This study was conducted to evaluate the durability of the SAPIEN XT when deployed in an explanted PERIMOUNT valve with moderate to severe calcification. A nominally deployed SAPIEN XT was used as a reference article.
Explanted surgical valves were selected from customer returned valves that met the criteria list below:
– Valve was stenotic and exhibited extensive calcification
– Frame was not noticeably damaged during explant
– Sewing band was intact such that the valve could be mounted to the test fixture
Figure 7A and Figure 7B show a representative test valve mounting technique, which displays the inflow and outflow view of a size 23 mm explanted PERIMOUNT valve prior to having a 23 mm SAPIEN XT valve deployment. Figure 7C and Figure 7D show the same inflow and outflow view after the VIV deployment of a size 23 mm SAPIEN XT.
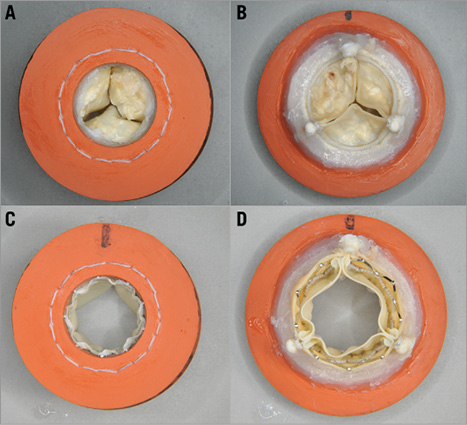
Figure 7. Mounting technique for an explanted calcified PERIMOUNT SHV with SAPIEN XT (sample shown is a 21 PERIMOUNT with a 23 SAPIEN XT). A) & B) Inflow and outflow of PERIMOUNT before SAPIEN XT implantation. C) & D) Inflow and outflow of VIV combination after SAPIEN XT implantation.
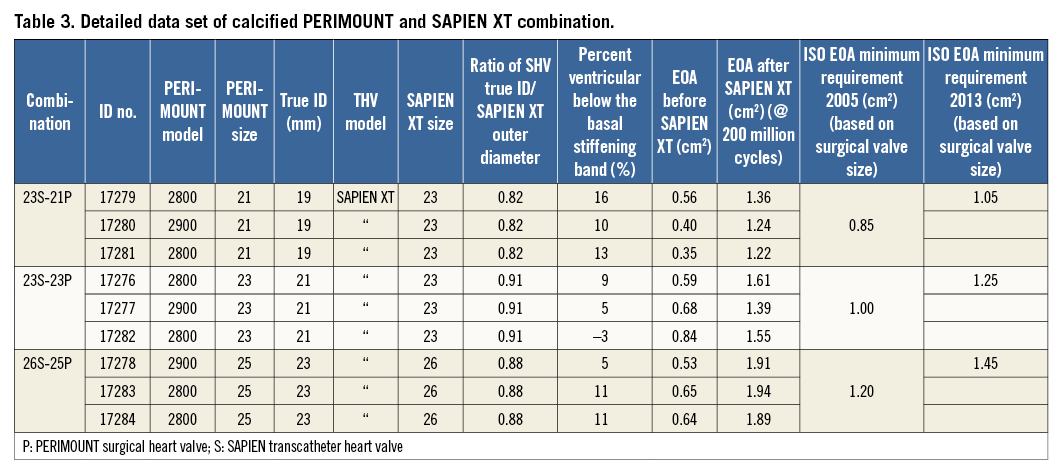
SAPIEN XT implantation was carried out using the NovaFlex+ delivery system in an identical fashion to the valves in the prior section with regard to depth and orientation (Figure 8, Table 3).
The test protocol was the same as that for the valves in the prior section.
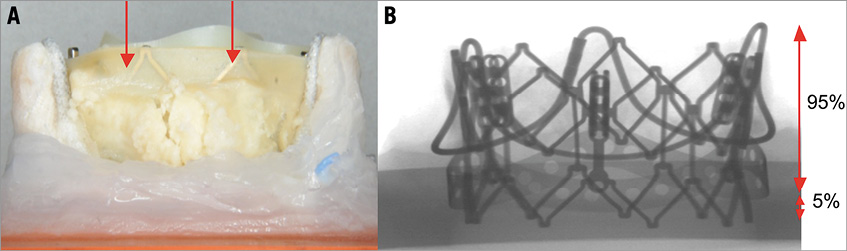
Figure 8. SAPIEN XT frame position within calcified explanted PERIMOUNT. A) Side view of a 23 PERIMOUNT with a 23 SAPIEN XT with the frame visible through the leaflets (red arrows). Deployment was performed to align the top of the THV with the top of the leaflets of the SHV. B) Fluoroscopic view of the same combination demonstrates that 5% of the SAPIEN XT frame is below the basal band, i.e., ventricular.
Results
PART A: IN VITRO VIV DURABILITY STUDY OF SAPIEN XT DEPLOYED IN A MAGNA EASE
All valves met the ISO 5840 minimum requirements, which are applicable to replacement valves, for effective orifice area (EOA) and regurgitant fraction at the initiation of the study, at the completion of 20 million cycles, and at the completion of 200 million cycles.
EOA
At 5 lpm and 70 bpm, the EOA for the 21 mm SHV – 23 mm THV VIV combinations was above 1.2 cm2 for all valves, the EOA for the 23 mm SHV – 23 mm THV VIV combinations was above 1.5 cm2 for all valves, and the EOA for the 25 mm SHV – 26 mn THV VIV combinations was above 1.8 cm2 for all valves, which were above the ISO requirements of ≥0.85 cm2 for 21 mm valves, ≥1.0 cm2 for 23 mm valves and ≥1.2 cm2 for 25 mm valves, respectively (Table 2). Reference transcatheter valves were included in this study. At the conclusion of the study at 200 million cycles, the EOA of the 23 mm reference valves exceeded 1.6 cm2 and the 26 mm reference valves exceeded 2.2 cm2.
REGURGITANT FRACTION
Regurgitant fraction for all valves was ≤7%, which met the ISO 5840 requirement of ≤10% for 21 mm and 23 mm valves and ≤15% for 25 mm valves, both initially and at 200 million cycles.
AWT DURABILITY TEST
The test and reference valves remained competent through 200 million cycles. All valves were able to maintain target peak differential closing pressures. No valve was removed from the study due to incompetence or other reasons. The test and reference valves displayed minimal wear on the leaflets. The VIV samples were all well seated. While some abrasions were observed on the valves, the abrasions did not progress to cause tears to the leaflets through the completion of 200 million cycles AWT. No fractures were observed under X-ray in the test and reference valves at the completion of 200 million cycles.
EMBOLISATION
There was no embolisation seen in any of the test articles throughout the testing. The largest migration of any SAPIEN XT within the Magna Ease valve was less than 1.2 millimetres. This occurred in a 25 mm SHV – 26 mm THV VIV combination within the first 20 million cycles and did not progress further.
PART B: IN VITRO VIV DURABILITY STUDY OF SAPIEN XT DEPLOYED INTO EXPLANTED CALCIFIED PERIMOUNT VALVES
All valves met the ISO 5840 minimum performance requirements, which are applicable to replacement valves, for effective orifice area (EOA) and regurgitant fraction at the initiation of the study, at the completion of 20 million cycles, and at the completion of 200 million cycles.
EOA
There was an improvement in EOA with THV deployment. Prior to deployment, the valves had an EOA of 0.44-0.70 cm2. Post deployment, the EOAs increased to 1.36-2.12 cm2, and these improved EOAs persisted for the entire study (Table 3).
At the completion of 200 million cycles, the test and reference valves were evaluated against the minimum performance requirement for the EOA as per ISO 5840. The test and reference valves displayed EOA values well above the minimum EOA requirement and met the minimum EOA acceptance criteria (Table 3).
REGURGITANT FRACTION
At the completion of the study, the regurgitant fraction for all valves was below the 10% maximum allowable in the ISO for 21 mm and 23 mm valves and also below the 15% maximum allowable in the ISO for 25 mm valves.
AWT DURABILITY TEST
Results show that all valves remained competent through 200 million cycles. Marked improvement in valve performance was observed when the SAPIEN XT was deployed into the stenotic valve. The test and reference valves displayed minimal wear on the leaflets. No fractures were observed under X-ray in the test and reference valves at the completion of 200 million cycles.
EMBOLISATION
There was no embolisation seen in any of the test valves throughout the testing. The largest migration of any SAPIEN XT within the explanted PERIMOUNT valve was less than 0.2 millimetres.
Testing for this study was conducted at the research and development laboratories of Edwards Lifesciences, Irvine, CA, USA.
Discussion
Since the first report of a successful VIV case, there has been a rapid increase in the use of THV to treat a failing SHV. There have been at least 16 stented and seven stentless valves manufactured and implanted in the aortic position in the last two decades13. Each valve is manufactured in multiple sizes to accommodate variations in patient population. If the label size is known, important dimensions can also be easily obtained12-14.
The majority of the experience in VIV has been with two THVs, balloon-expandable SAPIEN/SAPIEN XT and self-expanding CoreValve4-11. Recently, other emerging devices have also been used to treat SHV failures in the aortic position, namely the Portico™ valve (St. Jude Medical, St. Paul, MN, USA) and JenaValve (JenaValve Technology, Munich, Germany)17,18. THVs are designed to enable stability without sutures, hence they rely on relative oversizing to prevent acute or delayed embolisation19. THVs are manufactured in three to four sizes and are designed to function best when they are expanded fully and retain their end shape, which is mostly circular20.
Despite the many possible combinations, i.e., 16 stented aortic SHVs and four THVs, clinical results have been encouraging4-11. With increasing usage, however, the VIV treatment does seem to present some unique challenges. One of them is choosing a correct size of THV as undersizing can result in large paravalvular leaks and/or embolisation and oversizing may result in suboptimal function of a THV due to underexpansion21. Current recommendations for sizing of THV are an extrapolation of sizing recommendations by THV manufacturers for the native aortic valve7,10,13. Similarly, most of the functional data are available from individual case reports, and case series including VIV registries6-11. Although satisfactory, the outcomes in small label size valves have been alarming due to higher residual gradients. Mean residual gradients in the global VIV registry have been >20 mmHg in SHVs with ID <20 mm in 58% of patients treated with SAPIEN XT11. Although the causes for this could be multifactorial, it draws attention to the fact that VIV may not offer a good solution in small SHVs with currently available THV and SHV devices.
There is also a paucity of VIV in vitro data on issues such as the ideal placement of the THV device, migration/embolisation of the THV device, EOA after VIV (especially in small size SHV) and durability15,16. Hence, we chose to carry out in vitro testing with calcified explanted PERIMOUNT aortic valves and new Magna Ease valves. The rationale behind this was twofold:
The PERIMOUNT pericardial valve has been implanted for more than two decades in large numbers and hence clinicians will be faced with treating these valves with VIV.
The Magna Ease pericardial valve is currently being implanted due to haemodynamics and it has a large market share. By using a new valve we tried to simulate a worst case scenario after a VIV for each combination as there was absence of calcification or fibrin deposition which would have favoured secure placement.
DEPTH OF IMPLANT
As the SAPIEN XT is shorter in height compared to other THVs, it is important to ensure correct placement so that it is secure but also prevents SHV leaflet overhang. The sewing ring of the SHV is sutured to the aortic annulus at the time of surgical aortic valve replacement and can provide a reference level for secure anchoring14. In the Magna Ease and PERIMOUNT the basal band can also provide an additional anchor and visible reference level for placement14.
In the Magna Ease (supra-annular design), the level of the sewing ring is the same as the level of the basal band (Figure 1B). Thus the SAPIEN XT should be positioned below that level14. Based on bench testing, we had previously recommended placement of 10-15% of SAPIEN XT below the basal band14. During in vitro testing where attention was also paid to avoid leaflet overhang, we achieved 7-28% of SAPIEN XT lying below the basal band. We saw no THV embolisation. In the PERIMOUNT (Model 2800/2900), the level of the sewing ring is above the basal band (Figure 1A). Hence, we had recommended placement of the SAPIEN XT aligned to the bottom of the basal band14. This would ensure placement of the SAPIEN XT 10-15% below the sewing ring. In vitro testing similar to that of the Magna Ease resulted in placement of the SAPIEN XT 3-16% below the basal band. None resulted in embolisation. There was no SHV leaflet overhang.
The percentage variation was observed due to the relative height difference between the SAPIEN XT and Magna Ease or PERIMOUNT (Table 1), and also due to small variations, which can occur during simulated placement. As one cannot visualise the leaflet edge during a clinical case, it is safer to recommend 10-15% placement of the SAPIEN XT below the basal band for the Magna Ease and 0-5% for the PERIMOUNT as a guide for optimal placement. This is in agreement with the prior work published by our team14.
EMBOLISATION
Acute or delayed embolisation is another concern if the THV used is smaller than the true ID of the SHV. The test conditions simulated higher than physiological closing pressures and we did not observe significant movement in any of the combinations during these rigorous tests. The only combination where a 1.2 mm downward movement was observed was in one test sample of the Magna Ease 25 with a SAPIEN XT 26 (sample 17202, Table 2) where the SAPIEN XT 26 was 24% below the basal band of the Magna Ease 25. We think this is unlikely to happen in a clinical scenario as calcification of SHV leaflets helps to secure the THV6-11.
HAEMODYNAMICS
Global valve-in-valve registry data have suggested that, for small SHVs, the CoreValve might be better than the SAPIEN XT. Although these are real-world data, the two groups did not appear comparable, as comparison was based on SHV frame ID and not true ID11. True ID is the internal diameter measured after leaflet mounting12. Derivation of true ID for each SHV make and model has been previously described12. We have previously demonstrated that SHV design and choice of frame and leaflet materials may cause up to a 2 mm variation in true valve ID12. Thus, it is possible that valves in the SAPIEN XT group had smaller true ID than those in the CoreValve group despite having similar frame ID. In vitro data have demonstrated optimal EOA in both off-the-shelf Magna Ease 21 as well as calcified PERIMOUNT 21 mm when treated with a SAPIEN XT 23. The EOA remained adequate and complied with ISO requirements for the entire duration of the test in all three sizes. The experiment was set up before the new ISO guidelines of 2013 were published. EOAs remained satisfactory for all combinations, even when they were compared with the new ISO 5840-3:2013 guidance. Thus, at 5 lpm and 70 bpm, the EOA for the 21 mm SHV VIV combinations was above 1.2 cm2 for all valves (new ISO requirement ≥1.05 cm2), the EOA for the 23 mm SHV VIV combinations was above 1.5 cm2 for all valves (new ISO requirement ≥1.25 cm2), and the EOA for the 25 mm SHV VIV combinations was above 1.8 cm2 for all valves (new ISO requirement ≥1.45 cm2) (Table 2, Table 3).
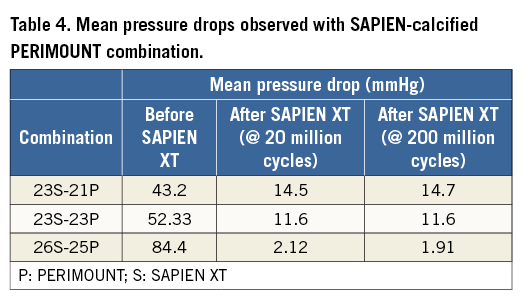
Azadani et al have carried out an elegant study to evaluate the use of a 23 mm “SAPIEN-like” THV within normal bioprostheses of equivalent or smaller orifice sizes16. Twelve valves designed to mimic the 23 mm Edwards SAPIEN valve were created using stainless steel stents and trileaflet pericardial valves. A custom-built pulse duplicator was used to measure the haemodynamic performance of these valves within 19, 21 and 23 mm Edwards pericardial bioprostheses. The transvalvular gradient, EOA and regurgitant volume were used to evaluate VIV therapy for each valve size. They observed acceptable VIV haemodynamics only in the 23 mm bioprosthesis after THV implantation, with no significant change in mean pressure gradient (5.93±0.87 to 8.27±1.19 mmHg, p=0.052) and EOA (2.13±0.15 to 1.79±0.15 cm2, p=0.053). In 19 and 21 mm valves, they observed severe and moderate stenosis, respectively. The mean pressure gradient increased from 16.18±2.20 mmHg to 45.53±12.54 mmHg (p=0.004) in 19 mm bioprostheses, and from 11.84±1.88 mmHg to 28.18±9.03 mmHg (p=0.004) in 21 mm bioprostheses. Furthermore, the EOA was reduced from 1.28±0.1 to 0.78±0.11 cm2 (p<0.001) in 19 mm valves, and from 1.51±0.15 to 1.01±0.19 cm2 (p<0.001) in 21 mm bioprostheses. They concluded that the patients with 19 and 21 mm Edwards pericardial bioprostheses may be poor candidates for VIV therapy with the currently available technology. We did not test 19 mm SHV, but haemodynamic results in 21 mm SHV with 23 mm SAPIEN XT were found satisfactory even when used in calcified SHVs with EOA >1.22 cm2 and reduction in the mean pressure gradient from 43.2 to 14.5 mmHg (Table 2, Table 3, Table 4). This difference could be due to the refined nature of the SAPIEN XT constructed by a highly complex manufacturing unit versus a hand-made SAPIEN-like device made by Azadani et al. This is supported by observation from a Nordic registry where clinically acceptable gradients were observed in 12 patients with frame ID less than 21 mm11.
REGURGITANT FRACTION
Other worries associated with incorrect sizing are PV leak due to undersizing and central leak due to incomplete expansion of a SAPIEN XT. Azadani et al observed an increase in regurgitant volume after VIV for all valve sizes16. Stent ID and true ID for 21 mm PERIMOUNT and Magna Ease are 20 mm and 19 mm, and for a 23 mm Magna Ease and PERIMOUNT the frame ID and true ID are 22 mm and 21 mm, respectively. We used a 23 mm SAPIEN XT to perform a VIV in these valves. For a 25 mm Magna Ease and PERIMOUNT with frame ID and true ID of 24 mm and 23 mm, we used a 26 mm SAPIEN XT. Hence, with every combination we had incomplete expansion of the SAPIEN XT of 1 to 4 mm. Nevertheless, we observed a very low percentage of leak, well within the limits set by old and new ISO standards.
DURABILITY
Although the attraction of VIV is its less invasive nature, there are concerns about long-term durability. The global VIV registry reported higher residual gradients when VIV was used in the treatment of small SHVs11. It can also be hypothesised that a greater mismatch between true ID and SAPIEN XT size can lead to incomplete expansion and leaflet folding, which may reduce the long-term durability. We found that leaflet durability and function were excellent in all combinations including calcified PERIMOUNT 21 mm and SAPIEN XT 23 mm combinations at the end of 200 million cycles. This is reassuring.
Limitations
The largest percentages of labelled sizes of SHVs implanted are 21, 23 and 25 mm, but 19 mm SHVs are not uncommon and are the most difficult to treat with a VIV due to their very small true ID (17 mm or less). According to the manufacturer’s recommendation, the smallest SAPIEN XT, i.e., size 23, can be used in the true ID diameter range of 18 to 22 mm, and hence we did not carry out the testing for the 19 mm surgical valve (true ID of 17 mm). Size 20 mm SAPIEN XT was not available at the time of testing although it is now commercially available. Larger label sizes were not studied as they accept a larger THV. We did not test the SAPIEN XT with SHVs from other manufacturers.
The haemodynamic tests are used to simulate hydrodynamic conditions but do not simulate physiology after human implantation. Tests were carried out at Edwards Lifesciences’ facility due to the costs involved in carrying out such a project, namely the cost of heart valves and test equipment.
Conclusions
To conclude, we have demonstrated secure placement, good haemodynamic performance and durability in the six VIV combinations which are commonly seen in clinical practice. Good haemodynamics and durability were also achieved even in SHV size 21 mm with frame ID of 20 mm and true ID of 19 mm when using a 23 mm SAPIEN XT transcatheter heart valve.
| Impact on daily practice Valve in valve is fast becoming a preferred modality of treatment for a degenerated surgical bioprosthesis. With a wide choice of multiple TAVI devices, it is important to use the right device, especially in small size bioprostheses. Our study provides robust early- and midterm outcomes as per ISO guidelines and will give confidence to the users to implant the SAPIEN XT in daily practice. Further, they can be assured of the durability of the newly implanted SAPIEN XT. |
Funding
This project was supported by an educational grant from Edwards Lifesciences, Irvine, CA, USA.
Conflict of interest statement
V. Bapat is a consultant for Edwards Lifesciences, Medtronic, Boston Scientific and St. Jude Medical. D. Chambers has no conflicts of interest to declare.

