
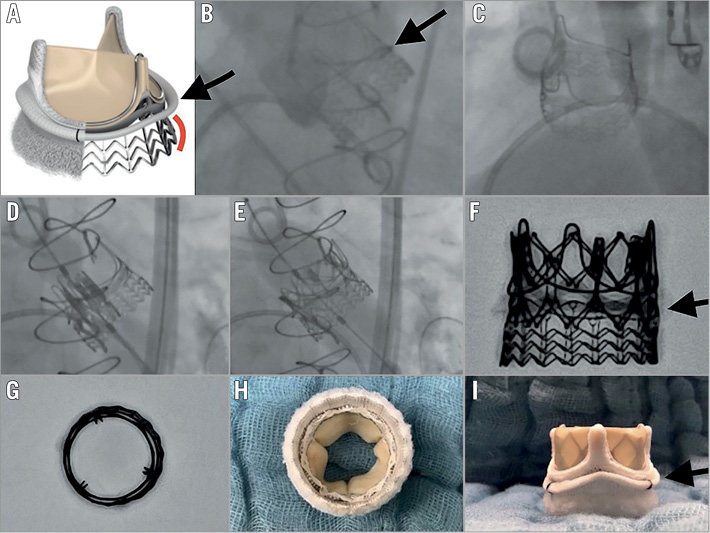
We report the first successful valve-in-valve (ViV) procedure implanting a 23 mm Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) into the novel rapid-deployment Edwards INTUITY™ Elite valve system (23 mm; Edwards Lifesciences) six years after surgery.
Rapid-deployment aortic valves (RD-AV) were recently implemented into clinical practice, showing good short-term and midterm results1. No experience is currently available for ViV procedures into this novel prosthesis. However, ViV procedures in other sutureless prostheses have already been reported, both after structural degeneration2,3 and in acute failure4 with good results in terms of safety and feasibility. The Edwards INTUITY Elite valve system is a supra-annular prosthesis with a stainless steel cloth-covered frame incorporated into the inflow aspect (Panel A-Panel C). The implantation requires three sutures, while the balloon-expandable frame establishes a seal within the left ventricular outflow tract (LVOT). Through a transfemoral approach, under fluoroscopic guidance (Panel D, Panel E), the Edwards SAPIEN XT was placed co-axial to the ring of the bioprosthesis (Moving image 1). It is crucial to obtain a proper alignment considering as a reference the bioprosthesis’ surgical ring (Panel A, Panel B, Panel F-Panel I, arrows indicating the ring in the lateral view) and not the stent frame of the valve, which lies within the LVOT (Panel A, red marker). We recommend using the sizing provided for the Magna valve5 (Edwards Lifesciences) also when using an INTUITY Elite valve due to the fact that the valves are very similar and the inner diameters are comparable. Therefore, a 23 mm Edwards SAPIEN XT valve was chosen.
After the primary deployment, a remaining moderate aortic insufficiency was observed. After post-dilatation, a trivial to mild regurgitation without significant haemodynamic effect was assessed (Moving image 2). No coronary obstruction was observed. The mean post-procedural gradient was 13 mmHg and remained below 15 mmHg at three-month and one-year follow-up. The patient was discharged three days after the procedure. Currently, this is the first patient with a degenerated RD-AV among more than 500 patients implanted up until now in our centre. The most important finding is that the anchoring of the SAPIEN XT valve in the INTUITY Elite prosthesis was achieved without problems. No change in the conformation of the INTUITY Elite stent frame occurred during implantation (Panel G, Panel H). Therefore, TAVR ViV procedures are technically feasible in patients who previously received an Edwards INTUITY Elite aortic valve prosthesis.
Conflict of interest statement
M. Andreas received a research grant and travel costs from Edwards Lifesciences. G. Laufer received consulting fees from Edwards Lifesciences. I. Coti received travel costs from Edwards Lifesciences. J. Kastner received speaking fees from Edwards Lifesciences.
Supplementary data
Moving image 1. Transcatheter valve implantation.
Moving image 2. Post-implant angiogram.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Transcatheter valve implantation.
Moving image 2. Post-implant angiogram.

