Abstract
Aims: Experience with transcatheter valve-in-valve implantation in a failing bioprosthetic tricuspid valve is very limited. Fewer than 30 cases have been reported, and in most of them the Melody valve (Medtronic, Inc., Minneapolis, MN, USA) was used. With this case report and review of literature we sought to evaluate the safety and feasibility of the Edwards SAPIEN transcatheter valve (Edwards Lifesciences, Irvine, CA, USA) in valve implantation in the tricuspid position and to compare this intervention with the more established Melody valve implantation.
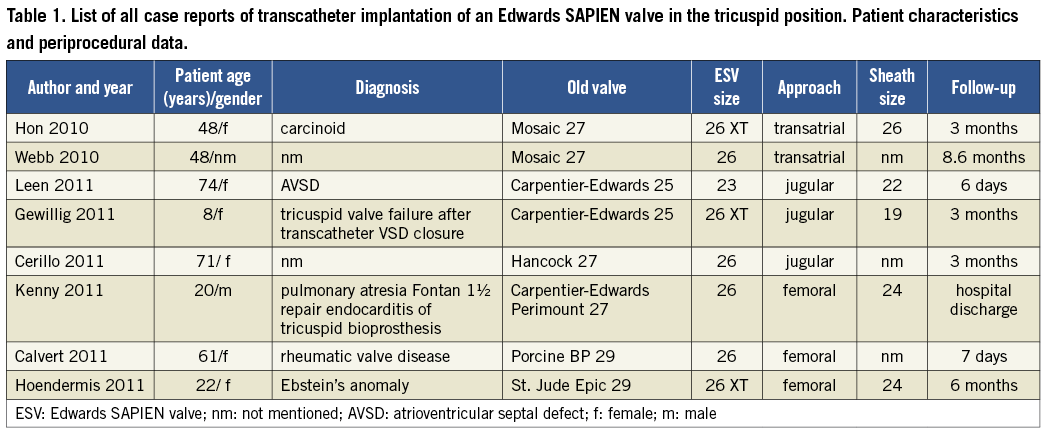
Methods and results: We describe one of the rarely reported Edwards SAPIEN valve implantations in a bioprosthetic tricuspid valve which is also the first in a patient with Ebstein’s anomaly. A review is presented of all eight case reports on Edwards SAPIEN valve implantations in tricuspid position. The procedure was successful in all cases. Valve performance after implantation was good and no complications were described. In only one procedure pre-stenting was performed. Transatrial, transjugular and transfemoral approaches have been used. The results are comparable to those of the series about Melody valve-in-valve implantation in the tricuspid valve. Mid-term follow-up data are not yet available for both valves.
Conclusions: Edwards SAPIEN valve implantation in tricuspid bioprosthetic valves is feasible and safe. Considering the available sizes of the Edwards SAPIEN valve, it may become the preferred prosthesis for valve-in-valve implantation in the tricuspid position in the future.
Introduction
Transcatheter valve implantation in different valve positions broadens interventional possibilities and is on its way to becoming an attractive alternative for multiple surgical re-interventions in a lifetime career of patients with congenital and acquired heart disease. After promising results of transcatheter valve implantation in the pulmonary valve position and the aortic valve position over the last 10 years1-4, recently reports have been published regarding the deployment of valved stents in the tricuspid valve although the data are limited. A transcatheter valve-in-valve implantation in a failing bioprosthetic tricuspid valve has been reported in fewer than 30 cases using two different valves: the Melody valve (Medtronic, Minneapolis, MN, USA) and the Edwards SAPIEN™ valve (Edwards Lifesciences, Irvine, CA, USA). The first case series regarding 15 Melody® valve (Medtronic, Inc., Minneapolis, MN, USA) implantations in bioprosthetic tricuspid valves was published recently5, while there are only a few reports on the Edwards SAPIEN valve in the tricuspid position. The Edwards SAPIEN valve is mainly used for aortic valve implantation.
Methods
We describe the first Edwards SAPIEN implantation in a tricuspid bioprosthetic valve performed at our own institution, in a patient with Ebstein’s anomaly. The patient’s written informed consent concerning the publication of this case was obtained. Furthermore, we searched in the online medical database “PubMed” for all articles published about Edwards SAPIEN valves implanted in the tricuspid position: all of them were case reports6-12. A review was performed of procedural details, valve performance and patient outcome of this rarely performed intervention. We identified eight cases including our own case. To put the two valves and procedures into perspective, the data were compared to available data about Melody valve implantation in the tricuspid valve.
Results
CASE REPORT
A 22-year-old female patient with Ebstein´s anomaly underwent tricuspid valve replacement with a Carpentier bioprosthesis in 2000 because of severe valve regurgitation and right ventricular dysfunction. Two years later, in 2002, the valve had to be replaced by a Carpentier-Edwards 33 mm prosthesis. In 2008, the valve prosthesis had to be replaced again due to severe calcification and stenosis of the valve. A St. Jude Epic 29 mm bioprosthetic valve (St. Jude Medical, Inc., St. Paul, MN, USA) was implanted surgically. In the following years, further rapid progressive degeneration of the new bioprosthetic valve occurred, and in 2011 the echocardiogram revealed a severely stenosed tricuspid valve with a peak gradient of 23 and a mean gradient of 12 mmHg, in combination with severe tricuspid regurgitation (Figure 1A, Moving image 1A). The right ventricular function was severely disturbed and the patient suffered from a decline in exercise tolerance with NYHA Class III symptoms and signs of chronic right heart failure with peripheral oedema, liver enlargement and periods of disturbed liver function tests. Diuretics were prescribed for treatment of decompensation. During a diagnostic catheterisation, a central venous pressure of 12 mmHg was measured. Pulmonary artery pressure, wedge pressure and pulmonary vascular resistance were normal. The mean gradient across the severely stenosed bioprosthetic tricuspid valve was 7 mmHg, and the tricuspid valve area 0.7 cm2. Angiography revealed a localised valvular stenosis with a diameter of 13 mm. The cardiac output was 3.1 l/min, and the cardiac index 1.9 l/min/m2. A further tricuspid replacement, in combination with a Glenn procedure to unburden the right ventricle, seemed to be the best surgical option. However, the risk of a surgical re-intervention was considered high because of the poor right ventricular function and the three previous thoracotomies. Furthermore, the lifespan of bioprosthetic valves in this young woman was obviously limited. Therefore, after full discussion with the patient, we finally decided to attempt a transcatheter valve-in-valve implantation in a hybrid setting with options for a bail-out procedure. Considering the vertical position of the tricuspid bioprosthesis (Figure 2A, Moving image 2A) we chose the transfemoral approach to reach a coaxial position of the transcatheter valve within the stenosed valve.
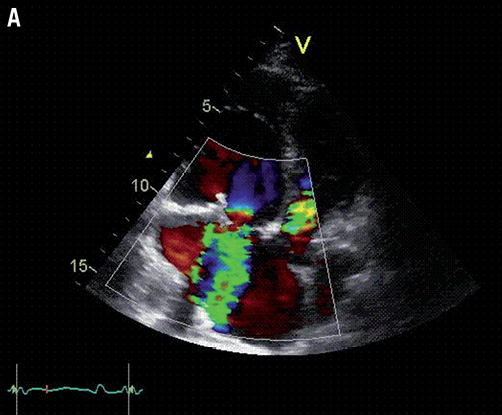
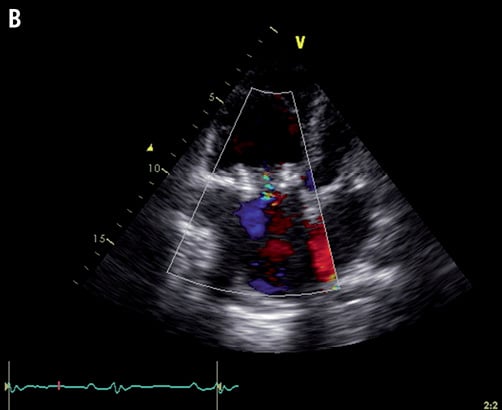
Figure 1. A) Transthoracic echocardiography showing the severely stenosed and regurgitant tricuspid bioprosthesis; B) Transthoracic echocardiography after intervention showing adequate position and function of the new transcatheter valve.
The procedure was performed under general anaesthesia. In the left femoral artery a pacemaker lead was inserted via a 6 Fr sheath to the left ventricle for rapid pacing. Through a 7 Fr sheath in the left femoral vein a pigtail catheter was advanced to the right atrium to perform angiograms simultaneously with valve implantation. Transoesophageal echocardiogram and angiography with a three-dimensional visualisation of the tricuspid bioprosthesis (DynaCT) provided us with adequate perioperative imaging. A 24 Fr sheath was inserted in the right femoral vein and a curved stiff guidewire (0.035-inch Amplatz extra stiff; Cook Medical Inc., Bloomington, IN, USA) was placed in the right ventricle. Valvuloplasty with balloon inflation to 23 mm was performed under rapid pacing. A 26 mm Edwards SAPIEN valve was then inserted via the RetroFlex™ Delivery Balloon System (Edwards Lifesciences, Irvine, CA, USA). The radiogenic ring of the stenosed biological prosthesis served as a landing zone, combined with the overlay of the DynaCT image. Finally, the new valve could be successfully implanted in the stenosed tricuspid valve under rapid pacing. The angiogram confirmed an excellent position of the valve (Figure 2B, Moving image 2B). The invasively measured mean gradient across the valve had decreased from 5 to 1 mmHg. There was no tricuspid regurgitation. The patient was monitored for two days on the cardiac care unit. Postprocedural echocardiograms on days two and four showed a good position of the new valve (Figure 1B, Moving image 1B). The peak gradient measured by echo Doppler had decreased from 23 mmHg preoperatively to 8 mmHg, and the mean gradient from 12 to 4 mmHg. There was only marginal tricuspid regurgitation. No complications occurred and the patient was discharged from the hospital at day five. The patient experienced significant clinical improvement. At three and six months follow-up, clinical and echocardiographic findings showed persistence of the results.
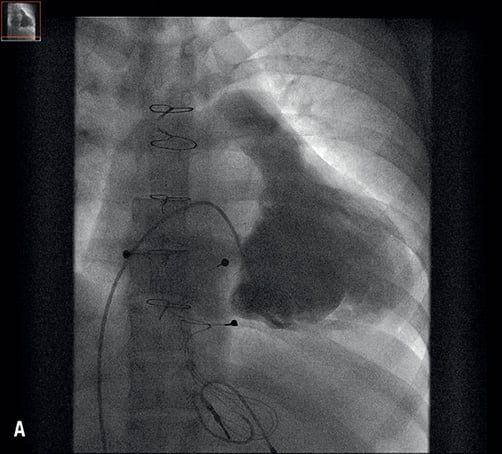
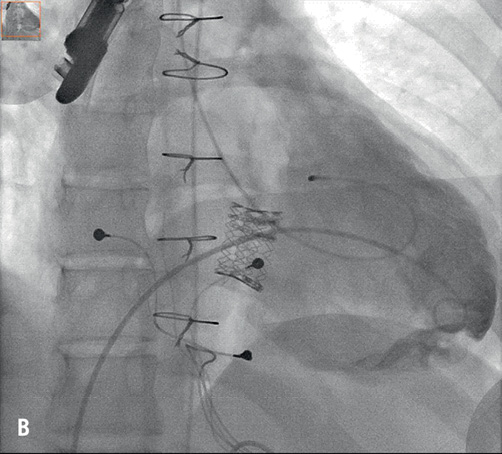
Figure 2. A) Fluoroscopy: right ventricular angiography revealing the severely stenosed and regurgitant tricuspid bioprosthesis; B) Fluoroscopy: final result after valve implantation.
EDWARDS SAPIEN VALVE IN THE TRICUSPID POSITION: REVIEW OF LITERATURE
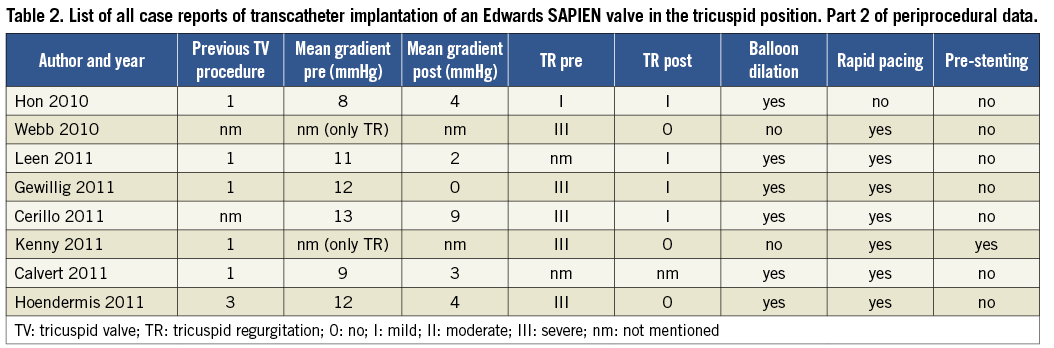
Including our own case there are now eight case reports published about an implantation of an Edwards SAPIEN valve in the tricuspid position in a patient with acquired or congenital heart disease6-12. Table 1 and Table 2 give an overview of patient characteristics and procedural details. All case reports describe valve-in-valve implantations in failing bioprosthetic valves (currently only animal studies report about percutaneous tricuspid valve implantation in native valves13). The age of patients ranged from 8 to 74 years (mean 44 years). Access to the heart was obtained by transatrial approach (by a hybrid approach through thoracotomy), transjugular approach and by transfemoral approach, respectively. Three patients had predominantly a stenosis of the valve, two had predominantly valve regurgitation and three had both. Pre-stenting was only performed in one patient with predominantly tricuspid regurgitation, and rapid pacing was performed in all but one patient. All procedures had good results without complications. The valve performance after implantation was good with a mean reduction of the tricuspid gradient from 10.8 to 3.6 mmHg in patients with predominantly tricuspid stenosis and no more than mild tricuspid regurgitation after valve implantation in all patients. These results are comparable with the results of the series with Melody valve implantations5. The reported follow-up was between five days and 8.6 months with a mean of three months.
Discussion
MELODY VALVE
The Melody valve, certified as an implant for the pulmonary valve14, was also successfully implanted in the tricuspid position for the first time in 2010. Three case reports documented that transcatheter Melody tricuspid valve-in-valve implantation can be performed safely and successfully13,15,16. Recently, the first series of percutaneous tricuspid valve replacement with Melody valves in 15 patients was published and reported good results5. Most patients had congenital heart disease, five of them with an RA-RV Fontan circulation. The valve was successfully placed in all patients, and the median follow-up was four months. In patients with predominantly valvular stenosis the mean gradient was reduced from 12.9 to 3.9 mmHg. In four patients balloon dilation took place before valve implantation. Pre-stenting was not described. Complications were one death in a patient with pre-procedural multi-organ failure, one with third-degree heart block requiring pacemaker implantation, and one case of endocarditis two months after implantation.
One important restriction of the Melody valve is its maximal diameter of 22 mm. Therefore, in the majority of tricuspid valves, larger devices will be necessary to guarantee a stable position.
EDWARDS SAPIEN VALVE
The Edwards SAPIEN system, developed for transcatheter valve implantation in patients with severe aortic valve stenosis, is available in sizes 23 and 26 mm for transvascular approach and also in size 29 mm for hybrid approach through thoracotomy. Although the largest size of the Melody valve (22 mm inner diameter) is comparable to the smallest Edwards SAPIEN valve (23 mm outer diameter) the whole range of sizes of the Edwards SAPIEN valve is expected to be sufficiently large for the annulus of most surgically implanted tricuspid valve prostheses. Disadvantages of the Edwards SAPIEN device are the need for larger sheaths and delivery systems, and the fact that it is more rigid and hardly tolerates deviation in positioning due to its short length. On the other hand the latter can also be regarded as a benefit as the valve does not protrude into the right ventricular outflow tract.
APPROACH
The tricuspid valve is often more directed towards the superior vena cava which suggests that the transjugular approach should possibly be preferred to obtain a better angle when positioning the stent, particularly when larger and more rigid devices such as the Edwards SAPIEN valve have to be deployed. However, the decision about the approach has to be taken for each individual patient separately and depends on the patient’s valve anatomy. In our patient with Ebstein`s anomaly a transfemoral approach was chosen taking into consideration the vertical position of the stenosed bioprosthesis. Including our case, three successful procedures of Edwards SAPIEN valve implantation via the transfemoral approach have been described. Our experience was that the steerable balloon catheter supported adequately the most challenging part of the procedure, which was to reach the coaxial position of the valve. In the series of Melody valve implantations5 the femoral vein was even the preferred approach, used in 11 of the 15 cases. Although the Melody valve does not come with a steerable delivery system, the angle from the inferior vena cava to the right ventricle by crossing the tricuspid valve seemed to be no problem, at least for this less rigid valve.
RAPID PACING
Positioning of the valve took place under rapid pacing in all but one of the Edwards SAPIEN case reports, but this may not be necessary in the low pressure system. For transcatheter pulmonary valve replacement, for instance, rapid pacing is not usually used. Hon et al6 described (and they are the only ones to do so) a successful procedure of tricuspid valve-in-valve implantation without rapid pacing. Probably the decision about the need for rapid pacing should depend on the extent of cardiac motion on angiogram rather than rapid pacing being used routinely.
BALLOON DILATATION AND PRE-STENTING
In all patients with valvular stenosis balloon dilatation took place before valve implantation. In the series with Melody valves5 predilation was performed in four of the 10 patients predominantly with stenosis. Pre-stenting was only described in one of the Edwards SAPIEN valve implantations in a patient with predominantly tricuspid regurgitation11. Pre-stenting was performed with a 39 mm AndraStent XXL (Andramed GmbH, Reutlingen, Germany). There was no case with pre-stenting described in the Melody valve group. This is in contrast to transcatheter pulmonary valve implantation where pre-stenting is performed in most cases to create a stable landing zone and to minimise the risk of fracture of the valved stent in the right ventricular outflow tract. However, this concern does not exist in the tricuspid position as the tricuspid valve is far away from any muscular tissue. Furthermore, most tricuspid valve prostheses are made with a ring whereas most pulmonary valve implants are without. These were probably the two main reasons why pre-stenting was not deemed necessary in the tricuspid position in a large majority of the cases.
LIMITATION
In the review we used accumulated case reports. This is a limitation as unsuccessful procedures are often not reported.
Conclusion
The Melody and Edwards SAPIEN valves appear to have comparable results concerning valve performance and safety. Together they offer a wide range of valve sizes and therefore they complement each other. We expect that in the tricuspid position the Edwards SAPIEN valve will be the valve of choice in most patients considering the standard sizes of tricuspid valves. However, mid- and long-term results are not available so far for both valves. Therefore larger case series with longer follow-up are needed.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement

