Abstract
Aims: Transcatheter implantation of valved stents (Melody and Edwards valves) for replacement of the pulmonary valve is currently an established procedure. We reviewed our experience on implantation of such valves in the tricuspid valve position.
Methods and results: Transcatheter valve implantation in the tricuspid position was attempted in five patients. Four patients had predominantly tricuspid valve regurgitation, two of whom also had tricuspid valve stenosis. All patients had severely symptomatic right heart failure. Patient median age and weight were 12 years and 50 kg (range six-43 years and 13-68 kg, respectively). All patients had a bioprosthetic valve already in place. The mean gradient across the tricuspid valve decreased from 12 to 3 mmHg after the procedure. Median procedure time and fluoroscopy time were 100 and 39 min (range 60-180 and 30-57 min, respectively). The patients’ functional class improved from NYHA Class III to II in three and from Class III to I in two patients during a follow-up period of 15-22 months.
Conclusions: Transcatheter replacement of malfunctioning bioprosthetic valves in the tricuspid position using valved stents is an attractive alternative to repeat surgery in high-risk or multi-operated patients. Longer follow-up and a larger number of patients are required to establish the long-term benefit of the procedure and freedom from reinterventions.
Introduction
Implantation of valved stents in the pulmonary valve position was first performed in 20001. These procedures have been performed mainly in right ventricular outflow tract conduits which have degenerated resulting in severe regurgitation, stenosis, or mixed disease. The majority have been with Melody® valves (Medtronic, Minneapolis, MN, USA) implanted in diameters of 18, 20 and 22 mm, or the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) implanted in larger diameters up to 26 mm2.
Transcatheter valve-in-valve implantation (TVIV) has been performed mostly for failed surgical bioprostheses in the aortic and the pulmonary position, rarely in the mitral position, and experimentally in the tricuspid position3,4. Implantation of valved stents in the tricuspid position has been performed in only a few isolated cases. Case reports have been published for the Melody5,6 and the Edwards SAPIEN valves7-10 and a multi-institutional study with inclusion of 15 patients11.
We report the second largest cumulative experience of five patients, who presented with severe tricuspid valve stenosis or regurgitation and underwent transcatheter implantation of valved stents in the tricuspid position.
Methods
PATIENTS
Tricuspid valve-in-valve replacement was performed in five patients, of whom three were children aged six, 11, and 12 years and two adults aged 17 and 43 years with median weight of 50 kg (range 13-68 kg). Details of the demographic data, underlying diagnoses and previous procedures are given in Table 1. All patients had one to three preceding open heart operations, including tricuspid valve (TV) replacement. The valves previously implanted were the 25 mm Carpentier-Edwards Perimount valve (n=4) and the 29 mm Carpentier-Edwards Perimount valve (n=1) (Edwards Lifesciences). The bioprosthetic valves were severely regurgitant in four patients, two of whom also had moderate to severe tricuspid stenosis (TS) with a mean gradient of 8-13 mmHg. One of the two adult patients had severe TS.
All children were symptomatic with exercise intolerance, hepatomegaly and mild peripheral oedema. The two adult patients, one with severe tricuspid regurgitation (TR) and one with severe TS, had shortness of breath, orthopnoea, severe peripheral oedema and ascites.
IMPLANTATION PROCEDURE
Informed consent was obtained from all patients. All procedures were performed in the cardiac catheterisation laboratory under general anaesthesia using transoesophageal echocardiography (TOE) to assess the valve function and the gradient before the procedure, as well as guiding the implantation during the procedure.
Although technical details regarding transcatheter implantation in the tricuspid position vary, the general approach for both Edwards SAPIEN and Melody valve implantation is the same and this is summarised below. Access was obtained from the right internal jugular or the femoral vein. Invasive blood pressure monitoring was performed through an 18 gauge cannula or a 4 Fr sheath in the femoral artery. Anticoagulation was achieved with 100 units/kg of heparin to maintain an activated clotting time (ACT) above 250 seconds. Right heart catheterisation was performed to assess the right ventricular (RV) end-diastolic pressure and the pressure gradient across the tricuspid valve. After haemodynamic assessment, an Amplatz extra-stiff guidewire (Cook Medical, Bloomington, IN, USA) was passed through a balloon-tip catheter and positioned in a lower lobe branch pulmonary artery, preferably the right. Angiograms were performed using a multi-track catheter over the guidewire to assess the RV size and the degree of TR. The size of the effective orifice of the bioprosthetic TV was determined by TOE and by using a sizing balloon (Figure 1). Balloon sizing had to be performed as some of the bioprosthetic valves had developed stenosis and therefore their effective opening was different from the original inner diameter.
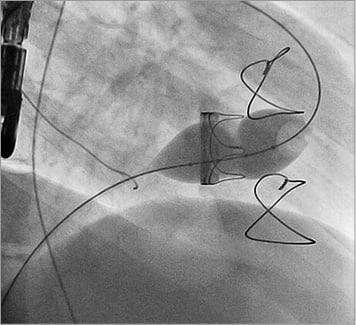
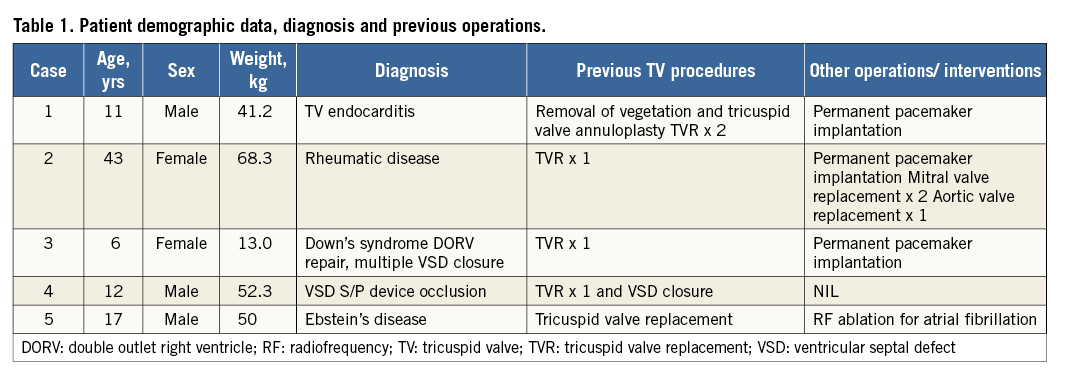
Figure 1. Balloon interrogation of the bioprosthetic tricuspid valve showing severe stenosis with prominent waist on the sizing balloon.
Predilation of the tricuspid valve with a high-pressure balloon in the patients with tricuspid stenosis was not required. We elected to pre-stent bioprostheses with a short landing zone, particularly during implantation of the Edwards SAPIEN valve.
After dilation of the femoral or jugular vein, and once the appropriate valve was prepared and crimped on the balloon, the short 18 Fr sheath was exchanged for the transcatheter valve delivery system (Figure 2). The inner and outer balloons of the balloon-in-balloon (BIB) catheter were then inflated to the pressure recommended by the manufacturer, apposing the valve to the walls of the bioprosthetic valve. After deployment of the valve, the delivery system was withdrawn (Figure 3). Repeat non-invasive and invasive pressure measurements and right ventricular angiography were performed after implantation (Figure 4). Post-dilation of the valves with higher pressure valvuloplasty balloons was not required.
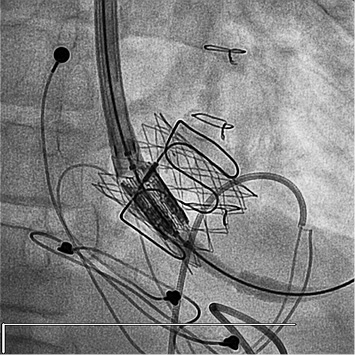
Figure 2. The tricuspid valve bioprosthesis has been pre-stented with a Palmaz 4014 stent (Cordis, Johnson & Johnson, Warren, NJ, USA) and the Edwards transcatheter valve delivery system is advanced into position.
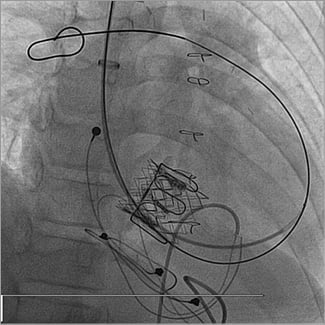
Figure 3. The Edwards valve appears well apposed to the walls of the bioprosthetic valve and the Palmaz stent.
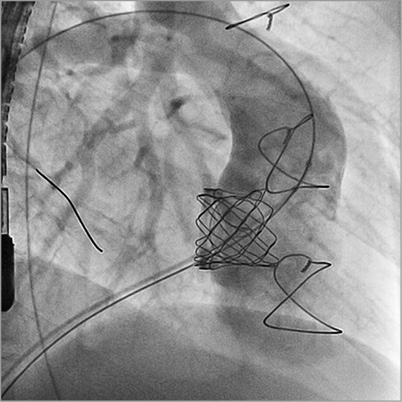
Figure 4. RV angiogram post Melody implantation showing no residual tricuspid regurgitation.
Haemostasis was achieved by using a Perclose AT device (Abbott Vascular, Santa Clara, CA, USA), manual pressure, or by the “figure of 8” stitch using size “0” Prolene® (Ethicon, Somerville, NJ, USA).
ANTICOAGULATION
All patients were started on aspirin on the evening of the procedure. Long-term antiplatelet therapy was continued for a total of six months.
Data analysis
The outcomes assessed included acute and chronic relief of TS or TR and related symptoms, survival and adverse events. For acute outcomes, variables of interest included acute efficacy and adverse events.
Results
Four patients received a Melody and one patient an Edwards SAPIEN valve (Table 2). Median procedure and fluoroscopy times were 100 min (range 60-180 min) and 39 min (range 30-57 min), respectively. The gradient across the tricuspid valve decreased from a mean of 12 mmHg to 3 mmHg (Table 2). No complications occurred during or in the immediate post-interventional period. The patients were all discharged within 24 hours after the procedure.
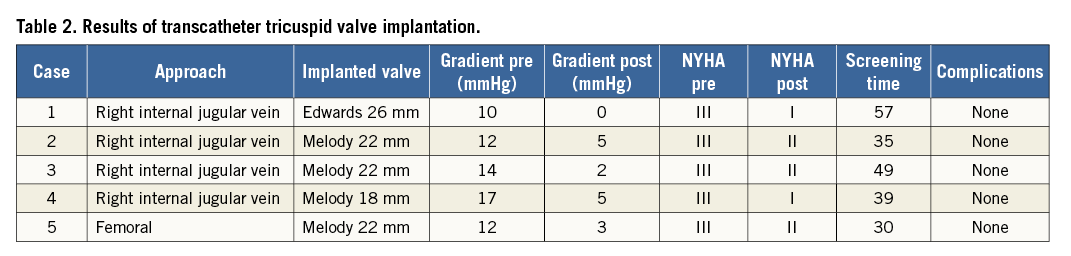
All patients improved clinically during the follow-up period with regression of the hepatomegaly and the peripheral oedema and ascites. The two children with mixed TV disease and the adult patient with predominantly TS had complete abolition of the stenosis. During follow-up of 15-22 months, TR improved to trivial in three patients and to mild in one patient. In the adult with predominant TS, the stenosis has not recurred.
NYHA Class improved from Class III to II in three patients and from Class III to Class I in two patients. No heart block or endocarditis occurred during follow-up. Diuretics were used in all patients post TVIV for six months and aspirin for six months.
Two patients with elevated RVED pressure on catheterisation have been placed on Lisinopril therapy post-TVIV.
Discussion
Bioprosthetic valve implantation in the great arterial or atrioventricular valves is often preferred over mechanical valves due to the lower risk of thrombotic or bleeding events and the avoidance of anticoagulants. However, bioprosthetic valves, especially in the tricuspid position, have a high rate of failure due to degeneration, which may cause TS, TR or mixed tricuspid valve disease3. A degenerated bioprosthetic TV may cause symptoms of right heart failure. Treatment options include aggressive medical therapy, percutaneous balloon valvuloplasty for stenosed valves or surgical valve replacement for severe TR. Percutaneous valvuloplasty may be an effective treatment; however, clinical experience with bioprosthetic TV stenosis has not been favourable due to the risk of inducing severe tricuspid regurgitation. Re-operation may carry significant operative risks of morbidity and mortality, especially in patients with severe symptoms12-14. Thus, an attempt at implanting a percutaneous valved stent is usually justified3.
There are two available stent valves suitable for implantation in the tricuspid position, the Melody and the Edwards SAPIEN pulmonary valve, both of which have been primarily used for pulmonary valve replacement. Recent publications report good results following implantation of one or other of these stent valves in the tricuspid position. It is important to know the type of the previous surgically implanted valve, as well as its external and internal diameter in order to select the most appropriate valve for percutaneous implantation. The mechanism of bioprosthetic TS may be pannus formation and intimal hyperplasia, which can be irregular within the inner surface of the valve. Therefore, balloon sizing was performed in all our cases to give us information on the optimum location of the constriction and therefore the landing zone, and about how the stented valve would behave during the inflation. Although there is a risk of thromboembolic events with the use of balloon catheters in valve-in-valve procedures in the aortic position, their use in the tricuspid position has been accepted due to the low incidence of calcification and the low documented risk of pulmonary embolism during balloon dilation of right heart valves, in particular because compliant sizing balloons are used.
Rapid ventricular pacing, commonly used for left-sided valves, is not usually required for TVIV implantation in the right heart valves. However, Calvert et al10 have reported transfemoral implantation of an Edwards valve with the aid of rapid coronary sinus pacing. The tricuspid valve bioprosthesis in their case was completely radiolucent, hence the procedure was performed without fluoroscopic landmarks and extra care had to be taken. We elected to pre-stent those bioprostheses with a short landing zone, especially when implanting an Edwards SAPIEN valve, because of its short stent length. It is easier to implant these when there is a safe landing zone, decreasing the chance of embolisation. If pre-stenting is not performed, then rapid pacing may be required to position the valve accurately. Rapid pacing may then need an access for pacing through the coronary sinus, left ventricle, or a pericardial approach.
Recent reports on TVIV implantation for failed bioprostheses in the TV on a limited number of patients show that percutaneous5-8,10,11 or perventricular9 implantation of a valved stent in the tricuspid position is feasible and low risk. So far these procedures have been on patients with operated congenital heart disease or infective endocarditis and rheumatic fever11. Nevertheless, complications have occasionally been reported. Third-degree heart block necessitating pacemaker implantation and one case of Melody valve endocarditis requiring valve removal two months post-implant have been reported so far. In addition, mild to moderate tricuspid regurgitation developed in one patient, in whom the valve was implanted with a 24 mm balloon11. Hence, care has to be exercised when over-dilating the Melody valve to more than 22 mm.
Although TVIV implantation in the tricuspid valve has been performed in patients with a permanent epicardial pacemaker system, it has not been attempted in patients who have an endocardial system in place with pacing leads through the tricuspid valve. Stenting has been performed successfully in severely stenosed or occluded superior vena cavas with pacing leads going through them, without impairment of the “trapped” pacing lead function post-stenting15. It is possible that the same principle would be applicable in the tricuspid valve position.
Future directions
Implantation of a valved stent in the tricuspid position is technically a relatively straightforward procedure. However, a recent report has highlighted early percutaneous valve failure in two patients who received a Melody and an Edwards SAPIEN valve. This may be related to the persistently high right ventricular end-diastolic pressure following the implantation, particularly in patients with borderline right ventricles, such as in patients with Ebstein’s anomaly16.
In addition, an alternative experimental treatment for patients with severe TR has recently been reported, whereby caval valve implantation is performed with an immediate abolition of IVC regurgitation resulting in midterm clinical improvement. It has therefore been suggested that in selected non-surgical patients caval valve implantation may become an additional therapeutic option to treat venous regurgitation and improve associated non-cardiac diseases17.
Conclusion
Transcatheter implantation of valved stents in the tricuspid position is an attractive alternative to repeat surgery in high-risk or multi-operated patients with TS, TR or mixed TV disease. Longer-term follow-up and a larger number of patients are required to establish the long-term benefit of the procedure.
| Impact on daily practice The use of bioprosthetic valves in the tricuspid position has a high rate of failure due to degeneration. To deal with the resulting stenosis or regurgitation, a valve-in-valve procedure with implantation of a Melody or Edwards SAPIEN valve should be considered. Initial results concerning the safety and efficacy of the procedure have been very encouraging. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

