Severe tricuspid regurgitation (TR) is associated with significant mortality1; heterotopic caval valve implantation (CAVI) is an emerging transcatheter solution for patients not deemed candidates for alternative therapies, yet the structural impact upon right heart dimensions is unknown2. TRICUS STUDY Euro is a non-randomised, single-arm, multicentre, prospective trial that recently demonstrated a high procedural success rate and significant improvements in both quality of life and functional parameters at 6 months with the TricValve (P&F Products and Features) transcatheter bicaval valves for the superior (SVC) and inferior vena cava (IVC). However, echocardiographic analyses at 3 months demonstrated a significant increase in right ventricular (RV) and right atrial (RA) volumes, as well as a decrease in RV function2. The present analysis focused on the computed tomographic (CT) volumetric imaging of the right heart to provide novel mechanistic insights into right heart remodelling at 6-month follow-up along with any propensity for caval valve leaflet thrombosis or issues with stent frame integrity.
All CT images were analysed and compared at the same phase in all cases. The quality was good or acceptable in 20 cases and poor in 6 cases due to poor contrast distribution; 3D reconstruction could be performed in 25 cases (92.6%). Leaflet motion was determined through a 4D-gated scan. Virtual reconstruction was performed with Mimics (Materialise), in order to understand the anatomical interplay (right to left ventricular ratio). Furosemide administration was recorded as a furosemide equivalent dosage (40 mg oral furosemide=1 mg bumetanide=20 mg torasemide).
Of 35 patients treated with the TricValve system2, 26 had baseline and 6-month follow-up computed tomography (CT). The mean age was 76.0±6.7 years, 84.6% were women, and the baseline tricuspid annulus plane systolic excursion was 13 mm. There was a statistically significant reduction of the RV mid-diameter at 6-month follow-up from 48.6±9.9 to 43.0±7.3 mm (p=0.001) (Supplementary Figure 1A) and a trend towards a decrease in tricuspid annular dimensions (mean diameter 49.2±6.0 to 47.6±5.4 mm; p=0.078) but both the SVC and IVC sizes remained similar. These findings are in agreement with the echocardiographic findings at 6-month follow-up (Supplementary Table 1). The 3D reconstruction demonstrated a significant decrease in the total RV volume at follow-up (180.5±77.8 to 147.4±67.7 cm3; p=0.037) with non-significant changes in the RA volume (289.1±123.5 vs 292.7±132.4 cm3; p=0.789) (Supplementary Table 1, Central illustration).
Regarding the safety analysis, none of the cases demonstrated SVC prosthesis thrombosis, whereas in 2 cases, stent thrombosis was detected in the IVC prosthesis. Both cases were located at the IVC-RA junction with normally functioning leaflets devoid of thrombosis (Supplementary Figure 2). Contrast leakage towards the IVC was detected in 5 cases; larger IVC dimensions and a marked angulation of its entrance into the RA were related to a greater risk of prosthesis leakage (Supplementary Table 2). At 6-month follow-up, 3 patients had died (8.6%), none of whom had significant paravalvular leakage, and 6 patients had been readmitted because of heart failure (17.1%), one of whom had significant paravalvular leakage that was successfully percutaneously sealed. The structural integrity of the prosthesis was preserved in all cases. Two cases of leaflet thickening were observed, one in an SVC and another in an IVC prosthesis, neither of these presenting with impaired leaflet motion.
TricValve, the first CE mark (European conformity)-approved CAVI system, demonstrated significant reverse remodelling of the RV and a strong tendency towards tricuspid annulus reverse remodelling – but not of the entire RA – despite the advanced stage of the patients enrolled in TRICUS STUDY Euro; to note, the mean furosemide equivalent dosage was reduced from 103±35 mg at baseline to 76±17 mg at 6 months, with no other relevant changes in medication. No stent fracture or leaflet thrombosis/early degeneration was detected at 6-month follow-up; however, 2 patients developed thrombi adherent to the stent frame that neither compromised leaflet/caval valve function nor were related to any clinically relevant adjudicated event. CAVI, through a reduction in RV volume overload and, less acutely, through a reduction in pulmonary arterial pressures, may induce a delayed reverse right heart remodelling. Indeed, systolic pulmonary pressure changed from 41.5±13.3 at baseline to 36.7±10.7 mmHg at 6-month follow-up, although this numerical decrease did not reach statistical significance. The detection of vena cava backflow in only 5 patients according to CT findings contrasts with the vena cava backflow reported in the 3-month echocardiographic follow-up in half of the patients2, suggesting that the endothelialisation process and/or the right heart remodelling might help to minimise this longer term.
Further patients with longer-term follow-up are required to establish this evolving concept.
These findings may have implications for selecting the most appropriate transcatheter tricuspid therapy for each patient. Orthotopic tricuspid valve replacement in patients with RV dysfunction poses a certain risk for acute RV decompensation and the need for inotropic therapy3. It is plausible that CAVI offers a better-tolerated alternative by gradually promoting reverse RV remodelling, utilising the RA as a reservoir to accommodate the acute afterload mismatch faced by the RV typically seen with acute TR abolition.
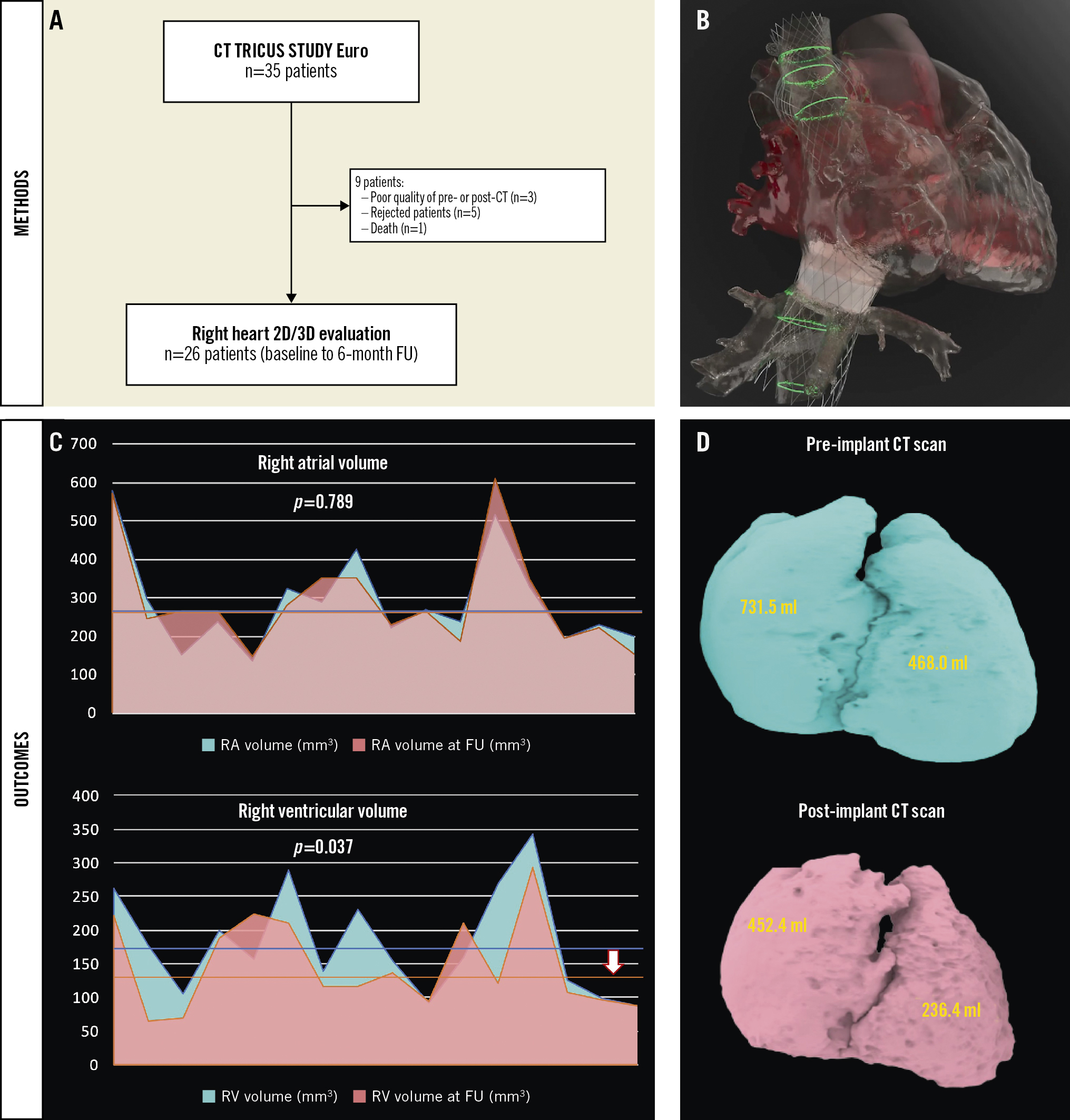
Central illustration. Right heart remodelling following TricValve implantation (CT analysis). Study flowchart (A). TricValve implant simulation (B) and main changes in right heart volumes, reflected as a patient-level analysis of right atrial and ventricular volumes (C) after 3D reconstruction (the arrow reflects the decrease in mean right ventricular volume). Case example of significant decrease in right chamber volume (D). 2D/3D: two-/three-dimensional; CT: computed tomography; FU: follow-up; RA: right atrial; RV: right ventricular
Funding
The participating institutions received a non-conditioned grant from P&F Products and Features for the TRICUS STUDY Euro study.
Conflict of interest statement
R. Puri serves as a consultant to P&F Products and Features and V-Dyne. I.J. Amat-Santos, I. Cruz-Gonzalez, A. Sánchez-Recalde, and O. Abdul-Jawad Altisent have served as consultants to P&F Products and Features. A. Iñiguez-Romo and R. Estevez-Loureiro received an institutional grant as principal investigators of the TRICUS STUDY Euro. J.L. Zamorano received an institutional grant for echocardiography corelab analysis. R. Estevez-Loureiro is proctor for Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

