Abstract
Approximately 4% of subjects aged 75 years or more have clinically relevant tricuspid regurgitation (TR). Primary TR results from anatomical abnormality of the tricuspid valve apparatus and is observed in only 8-10% of the patients with tricuspid valve disease. Secondary TR is more common and arises as a result of annular dilation caused by right ventricular enlargement and dysfunction as a consequence of pulmonary hypertension, often caused by left-sided heart disease or atrial fibrillation. Irrespective of its aetiology, TR leads to volume overload and increased wall stress, both of which negatively contribute to detrimental remodelling and worsening TR. This vicious circle translates into impaired survival and increased heart failure symptoms in patients with and without reduced left ventricular ejection fraction. Interventions to correct TR are underutilised in daily clinical practice owing to increased surgical risk and late patient presentation. The recently introduced transcatheter tricuspid valve interventions aim to address this unmet need. Dedicated expertise and an interdisciplinary Heart Team evaluation are essential to integrate these new techniques successfully and select patients. The present article proposes a standardised approach to evaluate patients with TR who may be candidates for transcatheter interventions. In addition, a state-of-the-art review of the available transcatheter therapies, the main criteria for patient and device selection, and information concerning the remaining uncertainties are provided.
Introduction
Tricuspid regurgitation (TR) is a common echocardiographic finding that is present in 70-90% of the general population1. While a trivial form is often seen in healthy individuals, moderate or severe TR has an age- and sex-adjusted prevalence of 0.55%, with a higher incidence in women and a strong age dependency2 - approximately 4% of subjects aged 75 years or more have clinically relevant TR2.
The development and successful results of transcatheter aortic valve implantation, followed by transcatheter therapies for mitral valve disease have opened a myriad of opportunities for transcatheter treatment of TR, a valvular heart disease that has traditionally been considered benign and often left untreated.
Chronic severe TR leads to volume overload and increased wall stress of the right ventricle (RV), which negatively contribute to detrimental remodelling and worsening TR. This vicious circle translates into impaired survival and increased heart failure (HF) symptoms in patients with and without reduced left ventricular ejection fraction3,4,5. Therefore, there is an unmet clinical need that requires prompt action. However, there remain many uncertainties and inconsistencies such as a non-systematic approach to assessing tricuspid valve (TV) disease, confusing terminology on anatomy and aetiology, as well as challenges in determining the mechanism and severity of TR and its consequences on the right chambers.
The present article proposes a standardised approach to evaluate patients with TR who may be candidates for transcatheter interventions. In addition, a state-of-the-art review of the available transcatheter therapies, the main criteria for patient and device selection, and information concerning the remaining uncertainties are provided.
ANATOMY OF THE TRICUSPID VALVE
The TV is the largest and most anterior cardiac valve with complex and variable anatomy6. Although its name infers the presence of three well-defined leaflets, numerous anatomical variations exist7,8. Differing terminology has been used6,7,8 and a simplified nomenclature is proposed (Figure 1),9 that has been derived from the analysis of 579 patients with various TR severity from 4 centres experienced in the assessement and treatment of TV disease. Based on the images provided, TV morphology could be determined in all but 4 patients (99%): 54% had type I, 4.5% had type II, 2.6% had type IIIA, 32.1% had type IIIB, 3.8% had type IIIC, and 2.4% had type IV. An in-depth understanding of the TV anatomy, in particular the number and location of supernumerary leaflets or scallops, is essential for procedural planning and may influence intervention outcome10.
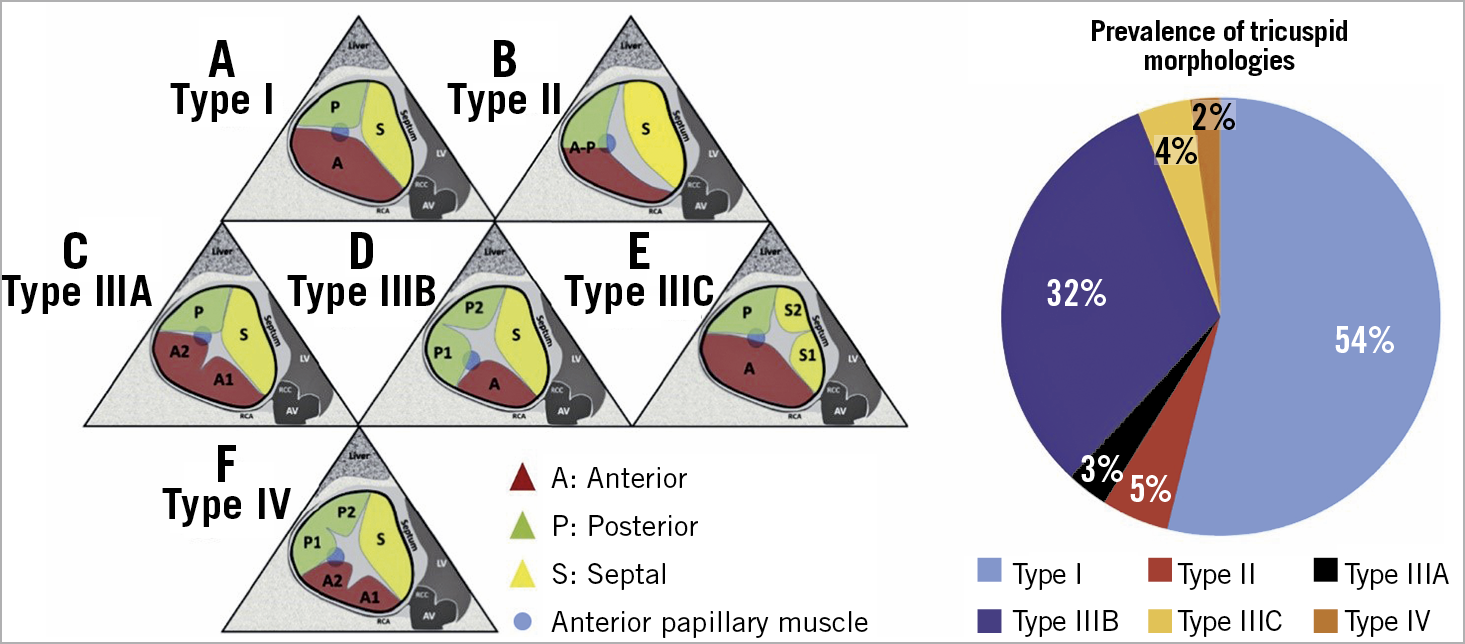
Figure 1. Proposed nomenclature for tricuspid valve classification. Left panel. Proposed nomenclature for tricuspid valve classification scheme (anterior papillary muscle [blue circle] defines separation of anterior and posterior leaflets). A) Type I: 3-leaflet configuration. B) Type II: 2-leaflet configuration. C) – E) Type III: 4-leaflet configurations. F) Type IV: 5-leaflet configuration. Right panel. Incidence of each morphology. A: anterior leaflet; AV: aortic valve; P: posterior leaflet; S: septal leaflet. Adapted from Hahn et al9, with permission.
Transoesophageal echocardiographic (TEE) imaging from the transgastric short-axis view (or three-dimensional [3D] volume-rendered equivalent) enables delineation of TV morphology. Steps to identify the leaflets are illustrated in Supplementary Figure 1.
CLASSIFICATION OF TRICUSPID REGURGITATION
Characterisation of the main morphologic and/or functional abnormalities resulting in TR is an essential aspect of transcatheter TV device selection. Primary TR results from anatomical abnormality of the TV apparatus and is observed in only 8-10% of patients with TV disease. Secondary TR (STR) is more common and arises as a result of annular dilation caused by RV enlargement and dysfunction due to pressure/volume overload as a consequence of pulmonary hypertension (PHT), often caused by left-sided heart disease, or atrial fibrillation (AF) with normal RV pressures (atrial/atriogenic or isolated TR). STR may also develop after left-sided valve surgery, probably due to silent ischaemic RV damage11,12. Implantation of cardiac implantable electronic device (CIED) RV leads provokes relevant TR in 20-30% of patients13,14,15, which frequently progresses over time16.
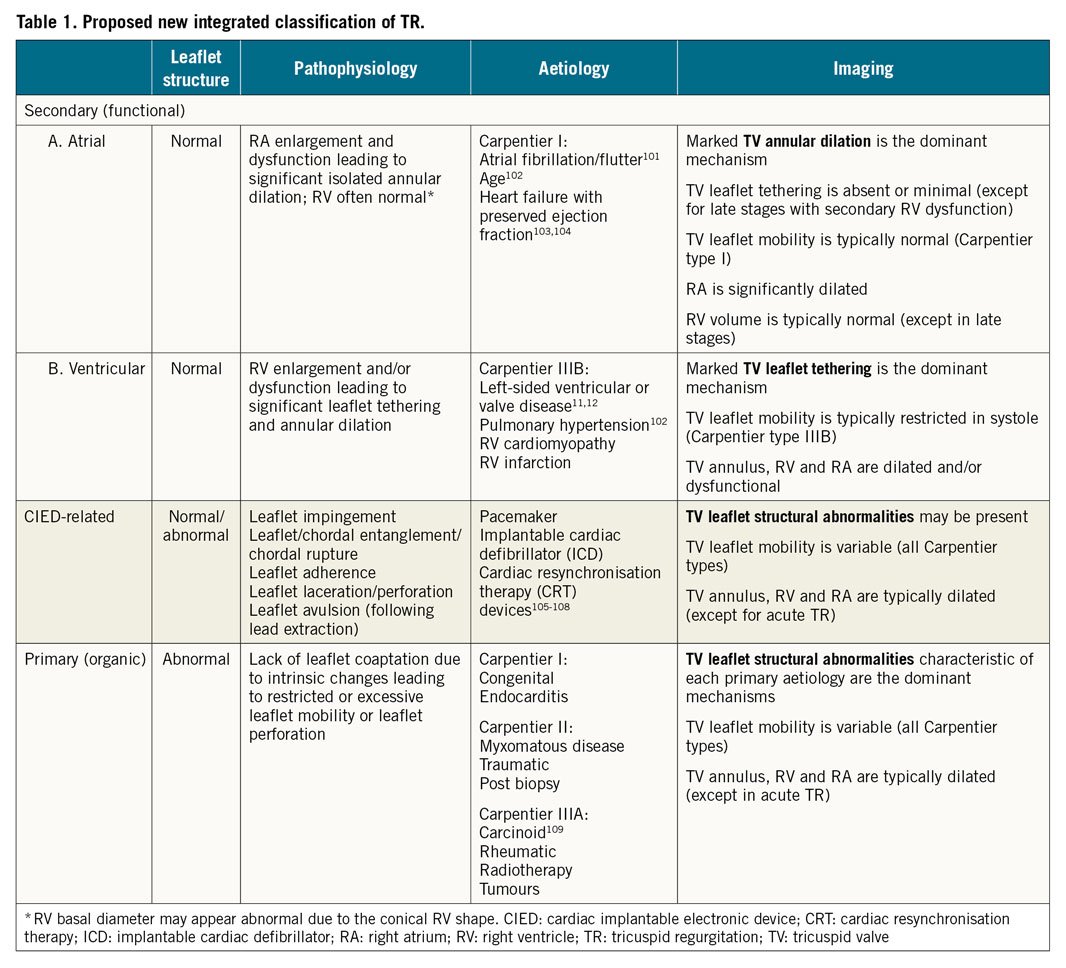
Carpentier’s functional classification of leaflet mobility was intended to guide mitral valve surgical repair or replacement and its application to the TV is less well established17. In addition to differences in TV leaflet mobility, patients with STR also demonstrate variable remodelling of the TV annulus, right atrium (RA) and RV secondary to the underlying pathology18. Definition of different TR groups is prognostically important since disease aetiology determines long-term outcomes. Accordingly, we propose a novel integrative classification of TR (Table 1) that accounts for the pathophysiology, imaging characteristics, clinical management and outcome, while recognising that differentiation of the initial aetiology based on valve and chamber morphology/function may be challenging as TV disease progresses.
DEFINITION OF DISEASE SEVERITY
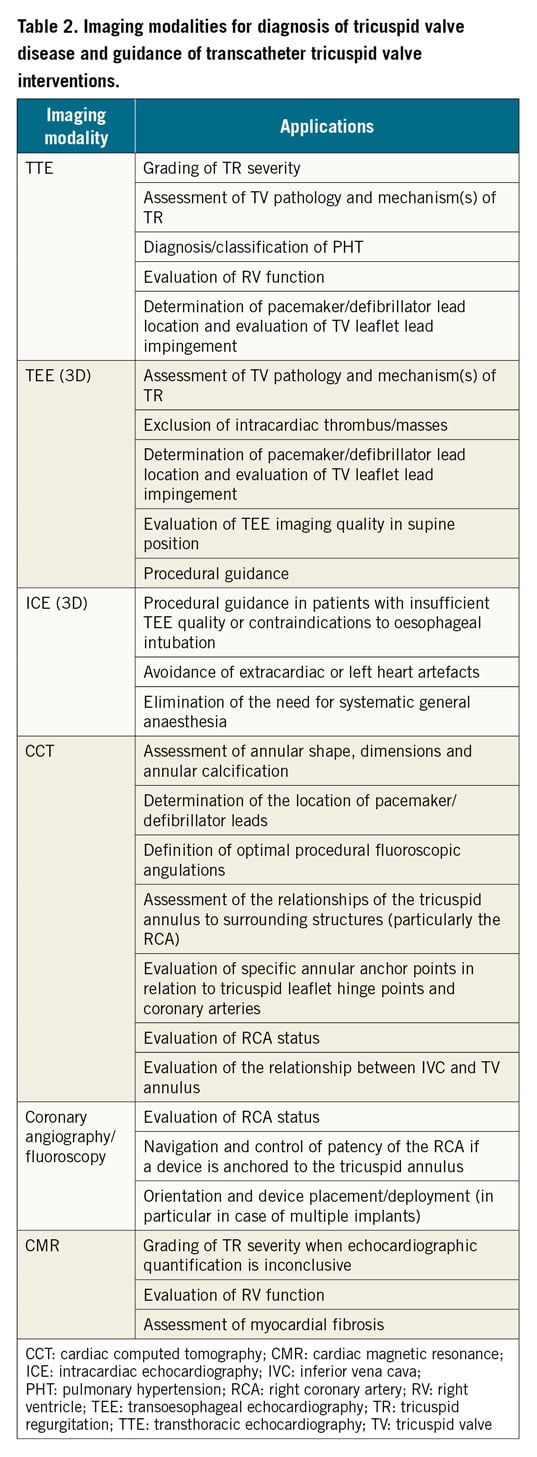
The functional anatomy of the TV apparatus can be evaluated by 3D transthoracic echocardiography (3D-TTE) and/or 3D-TEE, and severity of TR assessed using semi-quantative parameters of colour and spectral Doppler (Supplementary Figure 2),19. Additional advanced imaging may be of value when echocardiography is either of insufficient quality or inconclusive (Table 2). Specific signs of severe TR include wide systolic leaflet separation, hepatic vein systolic flow reversal demonstrated by pulsed-wave Doppler, and a triangular (early peaking) continuous-wave Doppler TR signal. RV and RA dilatation are supportive signs. The TR colour jet is not a measure of regurgitant volume, but is determined by jet momentum. Thus, whilst a small colour TR jet may reliably reflect trivial or mild TR and a very large jet is specific to severe TR, patients with PHT may demonstrate larger jets that overestimate TR orifice area. Furthermore, rapid equalisation of RA and RV pressures in severe TR may be associated with non-aliasing jets.
Quantitative measures of TR are therefore essential to define severity, including estimation of anatomical regurgitant orifice area by vena contracta measurement and quantification of physiological effective regurgitant orifice area (EROA) and regurgitant volume (RVol). A vena contracta ≥7 mm generally indicates severe TR19,20, although some studies suggest a threshold of 9 mm averaged from two orthogonal 2D views21. The TR coaptation zone is frequently non-circular and measures of vena contracta width relying on single 2D imaging may be inaccurate – planimetry using 3D colour assessment may therefore be conceptually more appropriate.
Accumulating evidence links EROA to outcome in various settings22,23 and current American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) guidelines define severe TR as an EROA ≥0.40 cm2 and RVol ≥45 mL20,24. However, since patients undergoing transcatheter TV interventions (TTVI) frequently have an anatomical regurgitant area several times greater than an EROA of 0.40 cm2, an extended classification to include “massive” and “torrential” TR (both associated with detrimental outcomes) has recently been proposed (Supplementary Table 1),23,24,25,26,27. Studies using cardiac magnetic resonance (CMR) quantitation suggest that patients with a TR regurgitant volume ≥45 ml or regurgitant fraction ≥50% have the highest risk of excess mortality28 (Table 2).
ASSESSMENT OF RV SIZE AND FUNCTION
Comprehensive RV assessment in patients with severe TR should be performed in a euvolemic state and include standard echocardiographic measures of RV size and function, and quantification of RV morphological, functional, and tissue remodelling (Figure 2, Table 2). Standard echocardiographic measures of RV size and function are listed in Supplementary Table 2,29. Assessment of RV strain using 2D echocardiography or CMR is less load dependent than tricuspid annular plane systolic excursion (TAPSE) in severe TR and more sensitive in the detection of early RV dysfunction and prediction of overall clinical outcome30. RV ejection fraction can be measured using various imaging modalities (CMR, 3D echocardiography, cardiac computed tomography [CCT]) but it fails to account for the relationship between RV contractility and afterload, and may therefore overestimate RV systolic function in severe TR. The TAPSE/systolic pulmonary artery pressure (SPAP) ratio, a non-invasive marker of RV-pulmonary arterial coupling, may overcome this limitation and its prognostic value has been demonstrated under several conditions31,32, including severe TR where a TAPSE/SPAP ratio <0.31 mm/mmHg indicates poor prognosis33. In a recent propensity matched analysis, patients with mid-range RV dysfunction (TAPSE 13-17 mm) appeared to derive the greatest benefit from TTVI34.
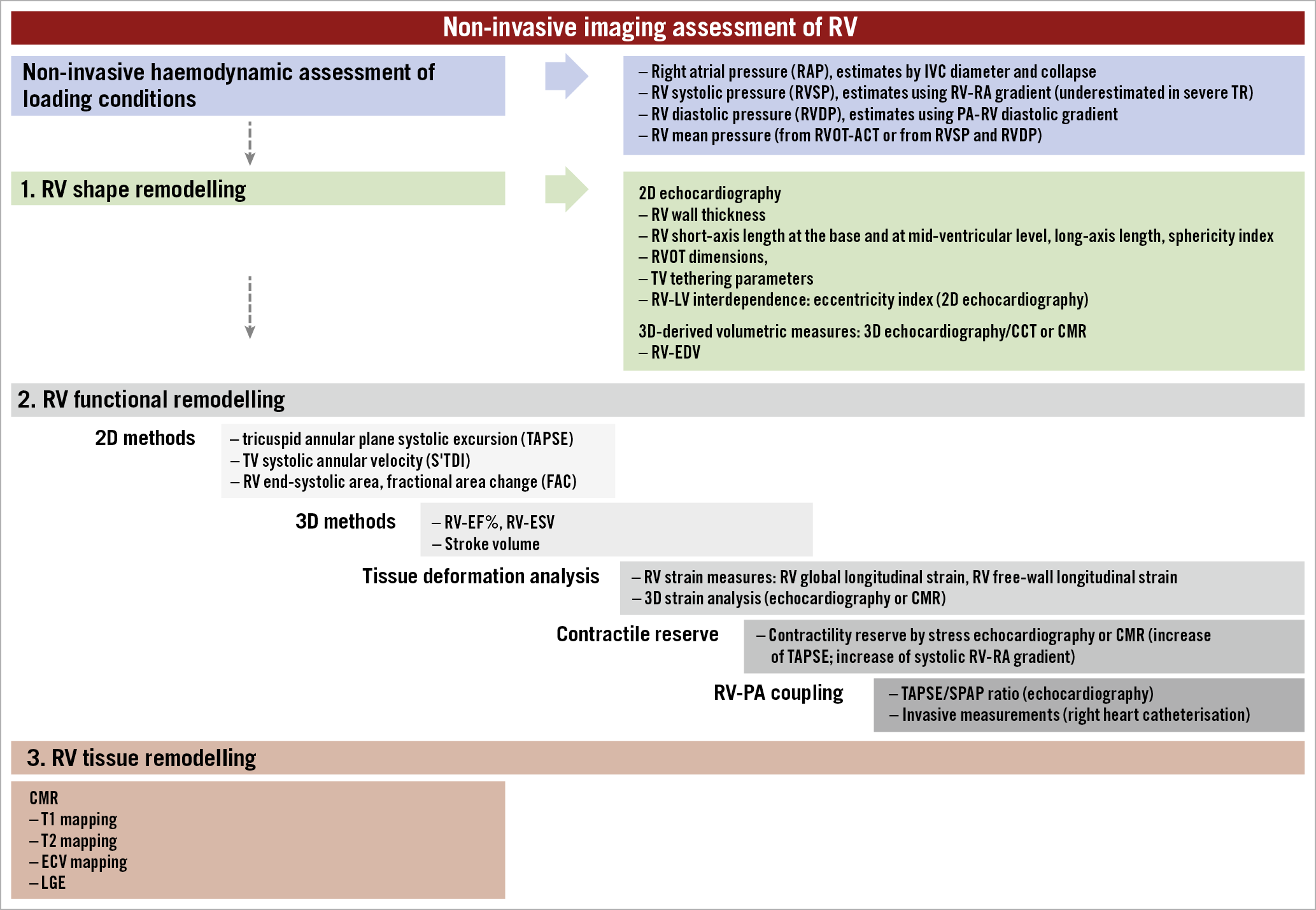
Figure 2. Imaging assessment of the right ventricle. 2D: two-dimensional; 3D: three-dimensional; ACT: acceleration time; CCT: cardiac computed tomography; CMR: cardiac magnetic resonance; ECV: extracellular volume; EDV: end-diastolic volume; EF: ejection fraction; ESV: end-systolic volume; IVC: inferior vena cava; LGE: late gadolinium enhancement; LV: left ventricle; PA: pulmonary artery; RV: right ventricle; RVOT: right ventricular outflow tract; SPAP: systolic pulmonary artery pressure; TV: tricuspid valve
The demonstration of contractile reserve in response to pharmacological or physical stress has prognostic relevance in patients with pulmonary hypertension and severe baseline RV dysfunction35,36,37; further studies are needed to explore the role of stress imaging in severe TR.
Right heart catheterisation is the gold standard for the assessment of the severity and mechanism of PHT, pulmonary vascular resistance, RA pressure/pulmonary capillary wedge pressure ratio, pulmonary artery pulsatility index, and the reversibility of PHT38,39. Moreover, an impaired afterload-corrected TAPSE to invasive SPAP obtained during right heart catheterisation and a discordant diagnosis of PHT (>10 mmHg difference between non-invasive and invasive SPAP) were independent predictors of worse outcomes (death, HF hospitalisation, and re-intervention) in patients with severe TR40.
Finally, detection of myocardial fibrosis by CMR41,42,43 has prognostic importance in RV failure and may help to define the optimal timing of intervention in severe TR.
PATIENT MANAGEMENT AND SELECTION
STR is associated with a variety of medical conditions and may present to a range of specialists (i.e., not only cardiologists). Efforts should be made to increase the clinical awareness of its consequences and emerging treatment options. All risk groups (those with left-sided heart disease, AF, previous mitral valve surgery, pre-capillary PHT, CIED RV lead) with symptoms/signs of congestive HF (jugular vein congestion, dyspnoea, peripheral oedema, renal failure, liver and gut congestion) should be specifically evaluated for the presence of significant STR. Initial consultation and echocardiography to confirm the diagnosis and assess TR severity and RV function should be followed by early referral to a centre with expertise in the treatment of TV disease where work-up may be completed by right and left heart catheterisation and advanced imaging studies (Table 2).
Currently, more than 90% of the patients with clinically relevant TR are not offered any treatment44, mainly due to the long-standing misconception that STR improves after treatment of left-sided heart disease, despite the fact that STR progresses in up to 25% of patients after open heart surgery11,12. Furthermore, relatively high mortality rates (8.8%-9.7%) have been reported after conventional surgery for isolated TR, usually as a result of late referral45,46,47,48. However, according to single-centre studies including younger and less sick patients compared to the TTVI cohorts, tricuspid surgery may be safe and effective when performed in experienced centres49,50.
Regardless of symptomatic status and clinical presentation, patients with severe STR should first be treated for the assumed underlying condition (e.g., restore sinus rhythm in patients with AF if feasible, optimise medical treatment of HF or PHT) followed by re-evaluation using the same imaging modality (ideally at the same imaging laboratory) (Figure 3, Supplementary Table 3). Repositioning or extraction of CIED RV leads can be envisaged in selected patients with disturbed TV leaflet motion. However, the efficacy of this procedure in reducing TR is uncertain and additional damage to the TV valve can occur51.
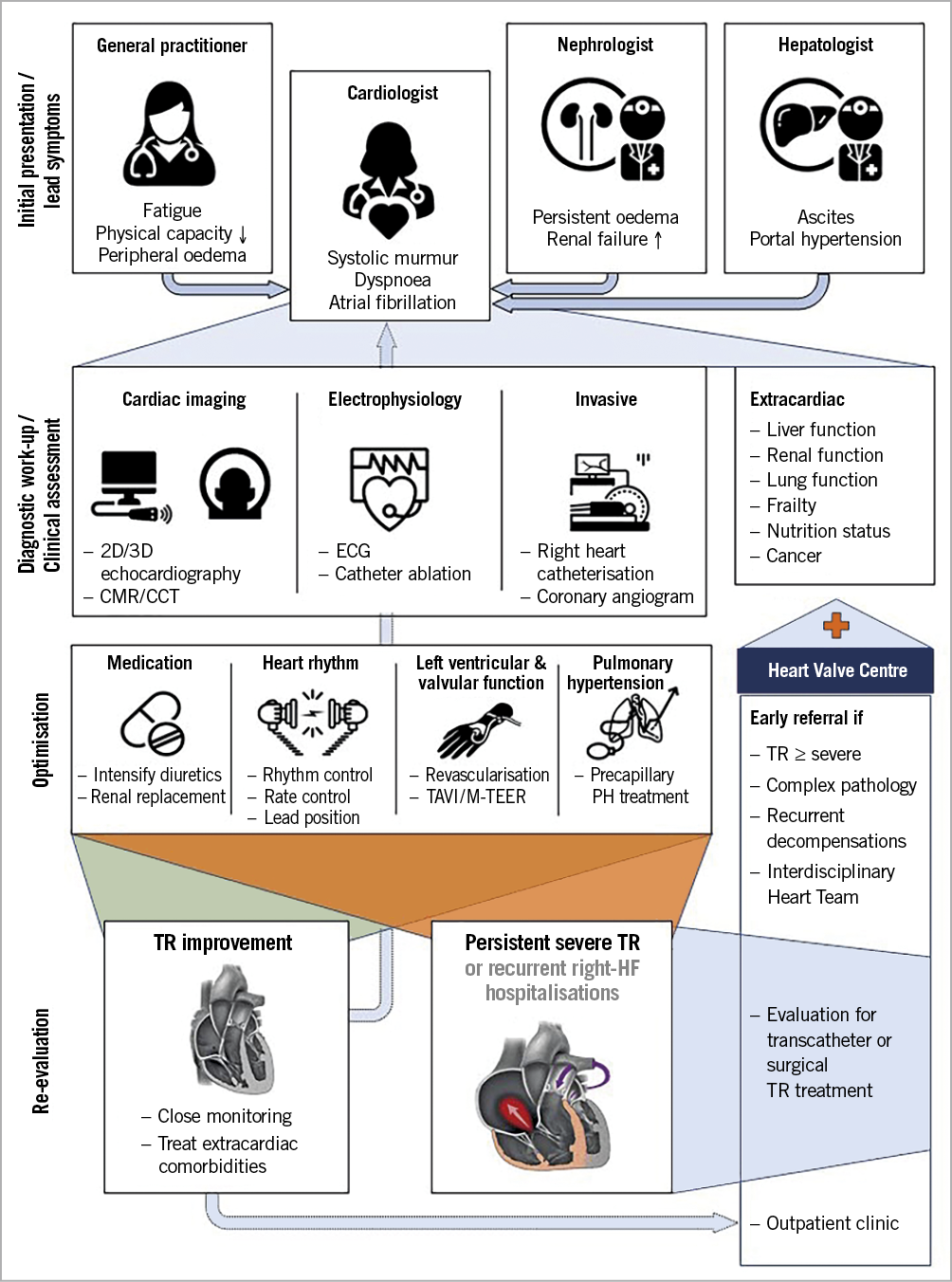
Figure 3. Care pathways for patients with severe tricuspid regurgitation. 2D: two-dimensional; 3D: three-dimensional; CCT: cardiac computed tomography; CMR: cardiac magnetic resonance; ECG: electrocardiogram; HF: heart failure; M-TEER: mitral transcatheter edge-to-edge reapair; PH: pulmonary hypertension; TAVI: transcatheter aortic valve implantation; TR: tricuspid regurgitation
The indication for any TV intervention and its timing should take account of multiple factors, including the patient’s clinical characteristics, disease severity, concomitant end-organ function and anatomical considerations (Figure 4). Those who remain symptomatic and fluid overloaded despite diuretic treatment with mild or moderate left ventricular impairment, preserved RV function, no evidence of pre-capillary PHT, and only mild/moderate renal and liver dysfunction may derive the greatest benefit from TV intervention (Figure 4, central column). Combined procedures may be considered in patients with associated mitral or aortic valve disease52 – a staged approach is often appropriate since TR and RV dimensions improve in about 40% of patients within three months of successful transcatheter treatment of mitral regurgitation53. Conversely, the procedure may be futile in candidates with end-stage HF, untreated pre- and post-capillary PHT or severe pulmonary fibrosis. Even if evidence is missing at this stage, advanced end-organ damage, i.e., terminal renal failure or manifest liver cirrhosis, need to be taken into account, in particular if the estimated life expectancy is less than one year (Figure 4, right column).
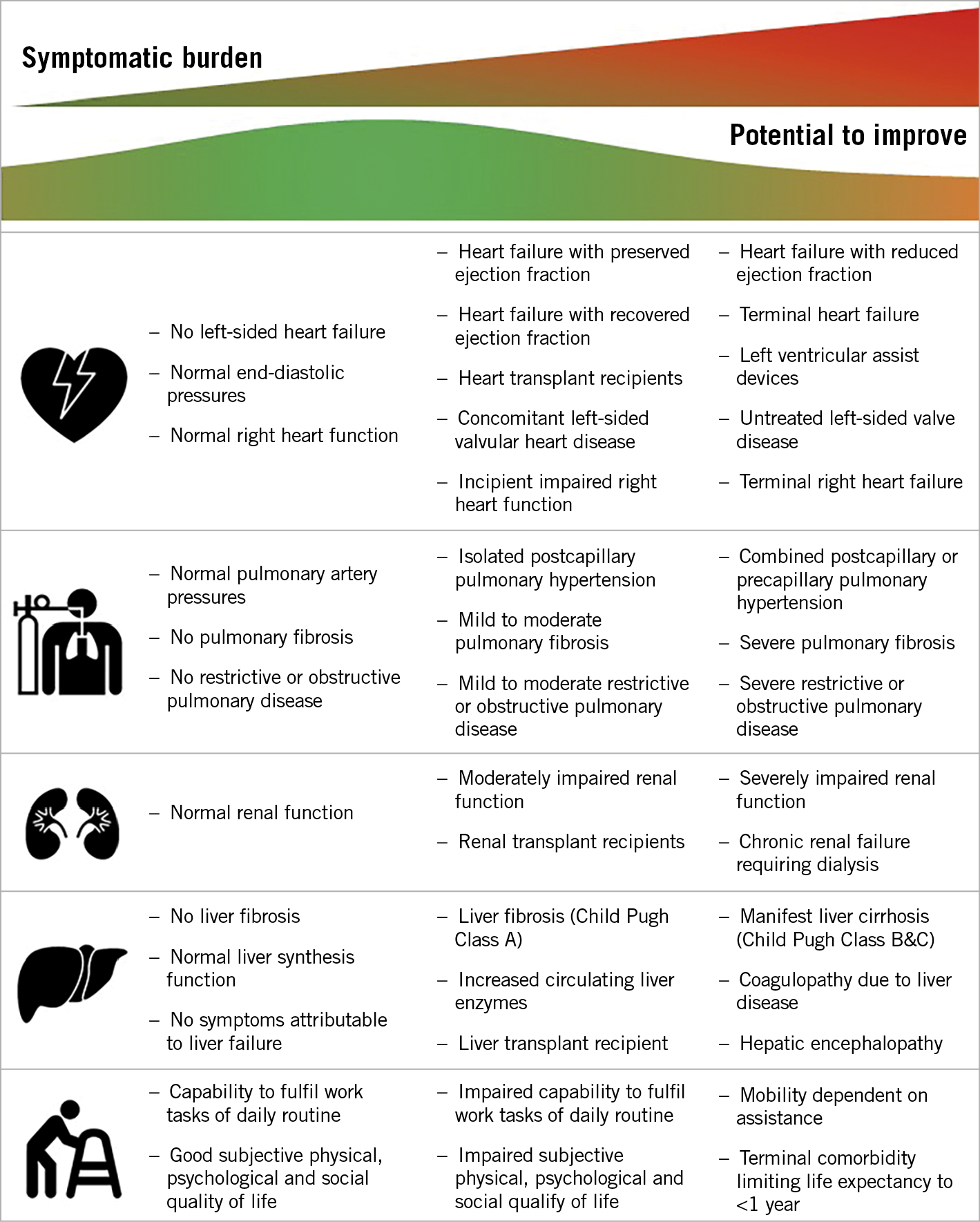
Figure 4. Clinical, anatomical and physiological factors suggesting a positive symptomatic response to tricuspid valve treatment.
Patients presenting with refractory hypervolemic state before the procedure may benefit from in-patient medical treatment optimisation, in particular a course of intravenous diuretics. This may favourably modify right chamber anatomy, annulus size, and reduce large coaptation gaps, therefore facilitating interventional treatment.
TRANSCATHETER TREATMENT OPTIONS AND DEVICE SELECTION
Current transcatheter treatment options mimic surgical techniques and include approved solutions in Europe, such as leaflet approximation, direct annuloplasty and heterotopic caval valve implantation, as well as not yet commercially available transcatheter TV replacement (TTVR) systems using orthotopic valve implantation (Figure 5). Growing evidence supports the use of TTVI in inoperable or surgical high-risk patients: mortality was lower following intervention using various devices compared to standard medical treatment in two propensity score-matched cohorts34,54, accompanied by reduction in the rate of HF re-hospitalisation (26±3% vs 47±3% p<0.0001) at one-year follow-up54. Confirmation of these findings in randomised controlled trials is needed.
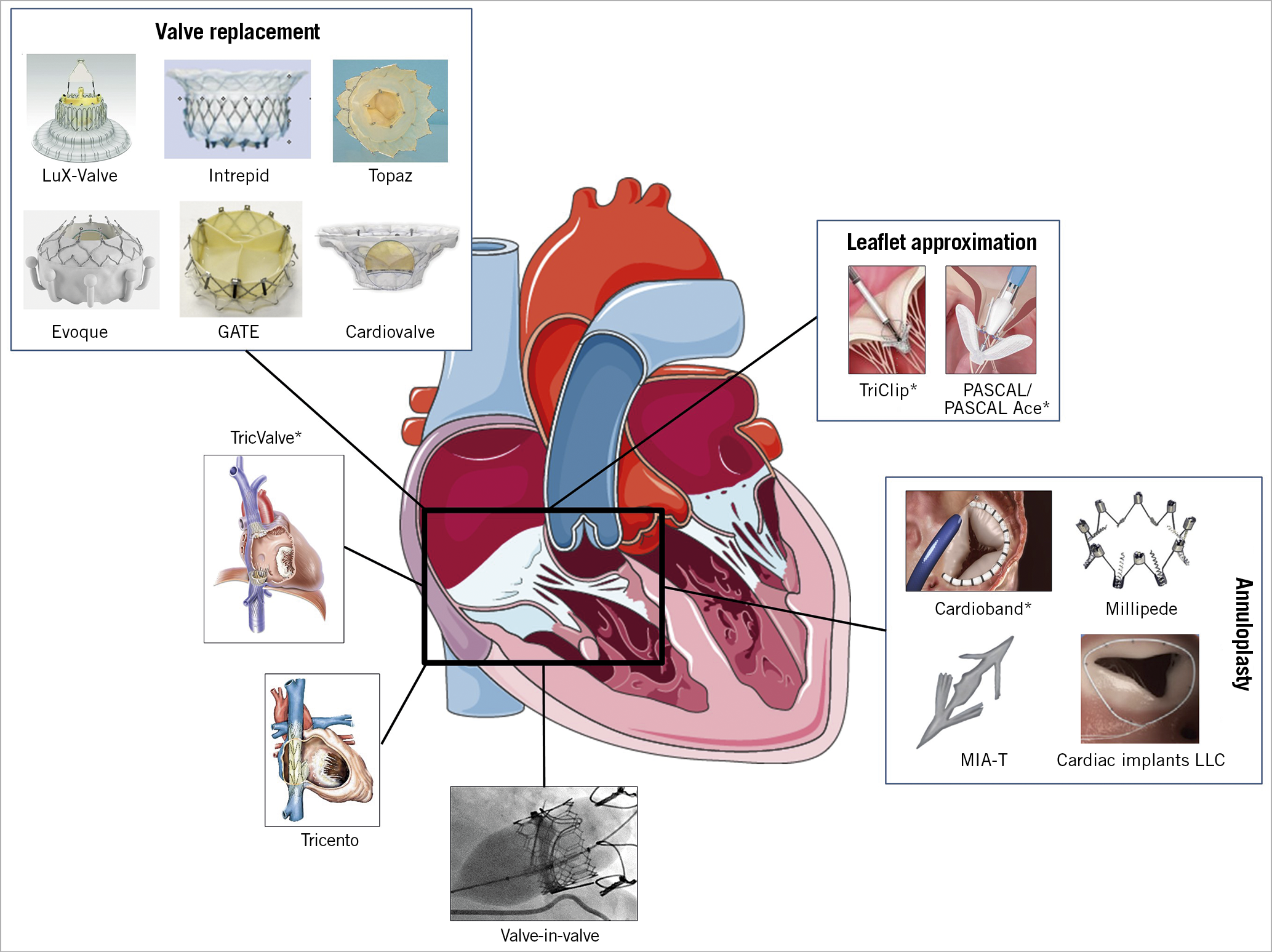
Figure 5. Transcatheter tricuspid systems that are approved or under clinical evaluation. * with CE approval.
Based on the aforementioned evidence, the 2021 Valvular Heart Disease guidelines of the European Society of Cardiology first give a IIb level C recommendation for transcatheter treatment of severe symptomatic TR in inoperable patients, while the importance of early referral of patients with TV disease, as well as the role of concomitant treatment of the TV during left-sided heart surgery, are reinforced55.
Compared to mitral procedures, TTVI presents several additional technical and anatomical challenges, including difficult visualisation of the TV apparatus, variable anatomy with thinner valve leaflets, and a large coaptation gap. Proposed criteria and an algorithm used for device selection are shown in Table 3 and Figure 6.
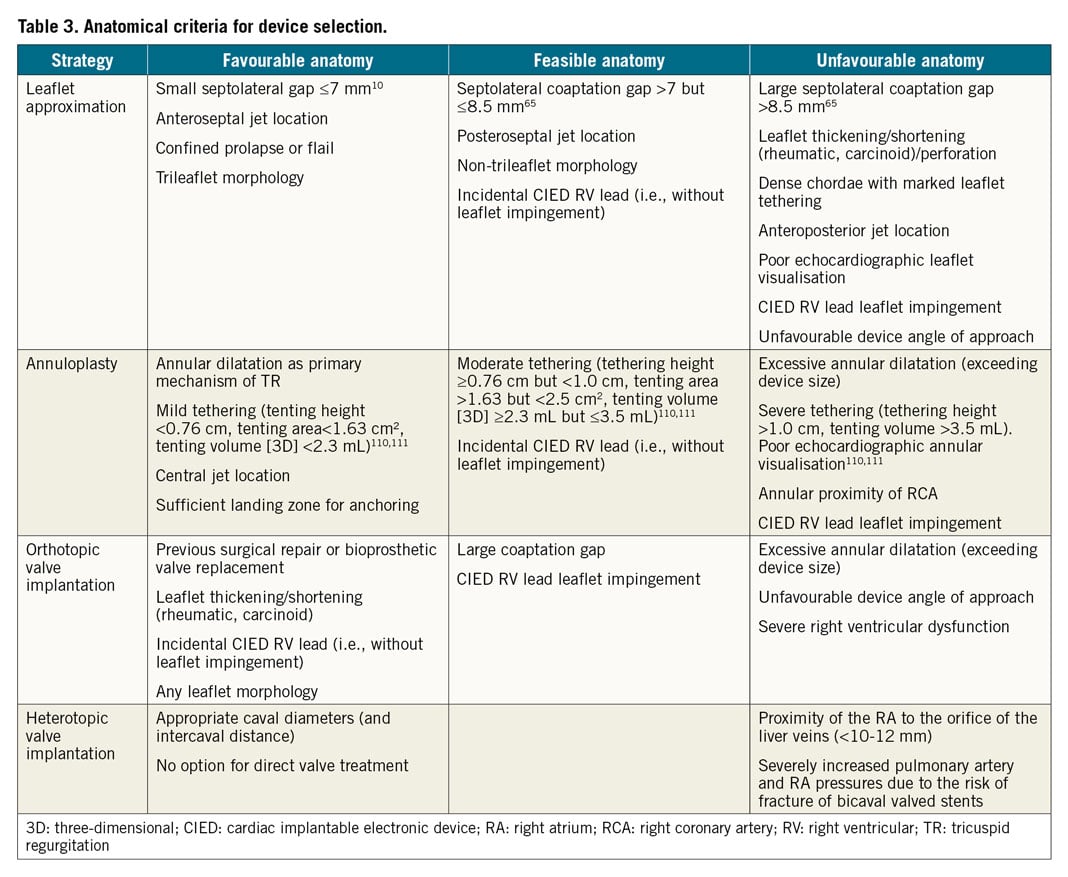
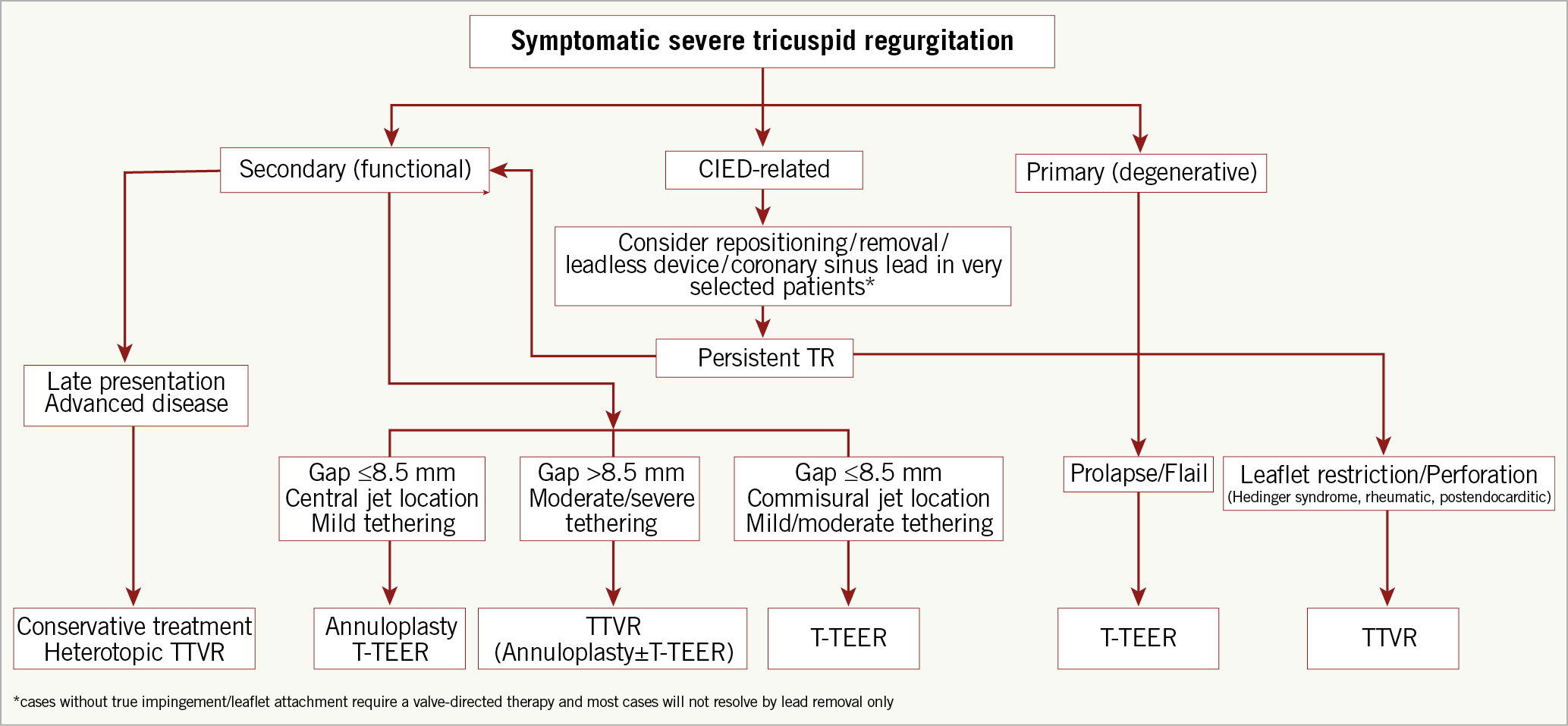
Figure 6. Proposed algorithm for the selection of TTVI systems. CIED: cardiac implantable electronic device; T-TEER: tricuspid transcatheter edge-to-edge repair; TTVR: transcatheter tricuspid valve replacement
LEAFLET APPROXIMATION
Tricuspid transcatheter edge-to-edge repair (T-TEER) using the TriClip™ (Abbott Vascular, Santa Clara, CA, USA) or leaflet approximation with the PASCAL systems (Edwards Lifesciences, Irvine, CA, USA) are approved in Europe for minimally invasive TV repair. These techniques are the most frequently used worldwide as a result of their safety, availability, and ease of use. Whilst initially performed “off-label” using the MitraClip® system (Abbott Vascular)56,57,58, a dedicated T-TEER device with a shorter curve guiding catheter and additional steerable plane of motion (septal to lateral), the TriClip, has been developed to facilitate TV access. In the core lab-adjudicated TRILUMINATE study, T-TEER in 85 prospectively enrolled patients (STR 84%; severe, massive and torrential in 29%, 26% and 37%, respectively) was associated with a durable reduction to moderate TR or less in 71% at one year, accompanied by symptomatic improvement (83% of the patients were in New York Heart Association [NYHA] Class I or II at 12 months), and 40% reduction in the rate of re-hospitalisation59,60. In addition, improvement of the six-minute walking distance by 31±10.2 metres and Kansas City Cardiomyopathy Questionnaire by 20±2.61 points were observed, along with a significant reduction of the RV and RA dimensions and improvement of the RV systolic function. Previous observational studies have demonstrated reverse RV remodelling61, improved cardiac output62, and reduction of liver enzymes in patients with documented congestion following T-TEER63. Early experience with the first version of the system identified an increased risk of single leaflet device attachement in comparison with mitral procedures (about 7% in the TRILUMINATE study). A large coaptation gap (>7-10 mm) and non-anteroseptal location of the TR jet have also been associated with procedural failure with the same version of the device10,64. Using the XTR implant with extended clip arms, a coaption gap ≤8.4 mm predicted successful reduction to moderate TR or less (Table 3),65. The latest TriClip Gen 4 iteration of the system offers four different implant sizes and controlled gripper actuation to permit optimised independent leaflet grasping.
Comparable results have been obtained with the PASCAL system in a compassionate use cohort30, as well as in a US-based early feasibility study66. Although less experience has been accumulated so far, the number of procedures has grown rapidly since introduction of the smaller PASCAL Ace (Edwards Lifesciences) device that facilitates navigation of the TV anatomy. The novel DragonFly™ transcatheter mitral valve repair device (Valgen Medical, Hong Kong, China) has recently been used successfully for mitral intervention67, and its use for TV repair is under current investigation.
ANNULOPLASTY
The Cardioband™ direct annuloplasty system (Edwards Life-sciences) obtained CE mark for the treatment of patients with severe symptomatic STR in 2018. In the European approval study (TRI-REPAIR), the device – consisting of a screw-anchored adjustable band – was successfully implanted in all 30 patients with sustained reduction of TR to moderate or less in 76% at 30 days, 73% at 6 months68 and 72% at 2 years69, and more than 80% of the patients in NYHA Class I/II throughout the follow-up period. Similarly, a 20% reduction in septolateral diameter was achieved in the post-market core laboratory adjudicated TriBAND study, translating into reduction of TR to moderate or less in 69% of patients at 30 days70. Of note, patients included in both studies had higher EROA at baseline compared to those in TRILUMINATE (TRI-REPAIR 0.79±0.51, TriBAND 0.76±0.48, TRILUMINATE 0.65±0.03), suggesting the inclusion of candidates with more advanced disease71. In another study including 60 patients of whom 51.7% had massive or torrential and 48.3% severe TR, 60.3% of patients had less-than-severe TR at discharge72. Particular care is required during deployment to avoid right coronary artery perforation or occlusion that occurred in 15% of the cases during the early experience (although transient deformation may not lead to clinical consequences)72,73. These results emphasise the need for further technical refinement along with careful patient selection and preprocedural planning.
Other annuloplasty techniques are under clinical investigations. The Millipede ring (Boston Scientific, Marlborough, MA, USA) has been implanted surgically in two patients74 and a transcatheter approach is in development. The MIA™-T system (Micro Interventional Devices, Newtown, PA, USA), a sutureless transcatheter annuloplasty system, is being investigated in a study (STTAR study) using both the surgical and transcatheter approach. Furthermore, successful implantation of a two-stage percutaneous annuloplasty system (Cardiac Implant LLC, Tarrytown, NY, USA) was reported in 202075.
TRANSCATHETER TRICUSPID VALVE REPLACEMENT
TTVR was first performed in 2017 using the GATE™ bioprosthesis (NaviGate Cardiac Structures, Inc., Lake Forest, CA, USA) that was introduced mainly via the transatrial surgical route using a 42 Fr catheter delivering an up to 54 mm stented valve76,77. Technical success was achieved in 26/30 (87%) consecutive patients with relevant conduction disturbances in 10% and conversion to open heart surgery in 5%. In-hospital mortality was relatively high (10%) in this early experience and inotropic support was required in 57% of patients, most probably due to transient RV failure78.
The LuX-Valve (Ningbo Jenscare Biotechnology Co. Ltd., Ningbo, China) is another 32 Fr system inserted via the transatrial access that can anchor to the septum and simultaneously grasp the anterior TV leaflet. Initial experience in 46 patients was associated with high technical success (97.8%) – one patient developed fatal RV perforation – and an in-hospital mortality rate of 13%79.
Surgical thoracotomy is associated with significant morbidity in patients with advanced disease, encouraging a move towards transfemoral systems. The EVOQUE bioprosthesis (Edwards Lifesciences) is delivered using a 28 Fr catheter and is available in three sizes (44, 48, and 52 mm). The system has been investigated on a compassionate use basis in 25 patients with successful implantation in 92%, reduction of TR to mild or trace in 100%, and no deaths, coronary complications, or valve migration. A permanent pacemaker implantation was required in 8%, and 76% of patients were in NYHA Class I/II at 30 days80. Preliminary results of the single-arm early TRISCEND feasibility study have been equally encouraging with an all-cause mortality rate of 3.8% at one month [Kodali S. TRISCEND study 30-day outcomes after transfemoral tricuspid valve replacement. EuroPCR 2021].
Transfemoral implantation of the Intrepid™ (Medtronic, Minneapolis, MN, USA) available in 42 and 48 mm sizes, the Cardiovalve (Cardiovalve Ltd., Or Yehuda, Israel), and the Topaz (TRiCares, Aschheim, Munich, Germany) transfemoral systems have also been reported in individual patients. Another self-expanding unileaflet stented bioprosthesis, the Trisol valve (Trisol Medical, Yokneam, Israel), is introduced via the jugular access and has recently been successfully used in humans.
Off-label transcatheter valve-in-valve implantation of the SAPIEN 3 aortic bioprosthesis (Edwards Lifesciences) is a safe and effective treatment option in patients with a degenerating surgical tricuspid bioprosthesis81, while suboptimal results have been observed after tricuspid valve-in-ring procedures82,83.
HETEROTOPIC CAVAL VALVE IMPLANTATION
Heterotopic caval valve implantation can mitigate symptoms related to TR and associated RV failure without treating its cause and is therefore a useful symptomatic treatment option in patients who are unsuitable for other transcatheter or surgical procedures. Conventional aortic balloon-expandable bioprostheses are too small in this setting and associated with deleterious embolic complications84. This has led to the development of the dedicated TricValve® (P+F Products+Features GmbH, Wessling, Germany) and TRICENTOM2M (MEDIRA GmbH, Balingen, Germany) devices. While TricValve features two valves implanted separately in the superior and inferior vena cava, TRICENTOM2M consists of a custom-made single valved stent linking both venae cavae. In their current iteration, both devices can treat patients with diameters of the inferior vena cava (IVC) up to 40-43 mm, while the distance from the RA junction to the hepatic veins needs to be at least 10 mm. Successful implantations of both devices have been reported in individual patients85,86,87, although recently described fractures of the TRICENTOM2M stent frame in patients with massive or torrential TR have led to design modification and adjustement of clinical selection criteria. Further evaluation in larger cohorts is required to understand the role and implications of this therapy better.
INTERVENTIONAL IMAGING
3D-TEE is essential for intraprocedural guidance during TTVI and the mid- and deep-oesophageal (RV inflow/outflow) and transgastric windows are of particular value (Figure 7). Beyond leaflet approximation, all other transcatheter techniques require dedicated preprocedural CCT assessement of the tricuspid annular and subvalvular anatomy, as well as RA and caval dimensions88. Implant simulation and real-time fusion of CCT, fluoroscopy and echocardiographic images may also assist in some procedures.
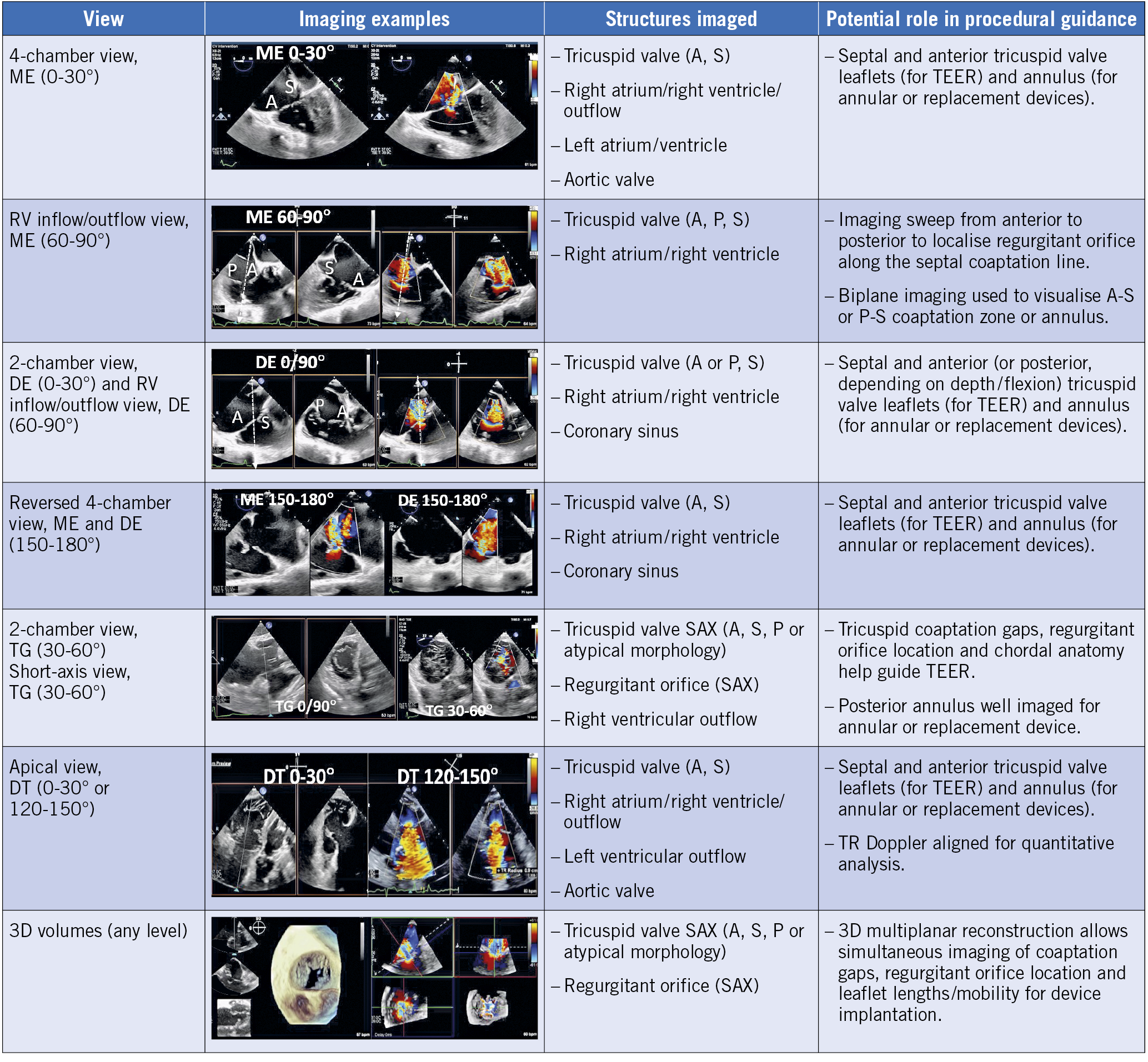
Figure 7. TEE views during transcatheter TV intervention. Deep oesophageal view for biplane imaging and acquisition of 3D volumes. The transgastric view is the only 2D view that allows simultaneous visualisation of all three TV leaflets. 3D: three-dimensional; A: anterior; DE: deep oesophageal; ME: mid-oesophageal; P: posterior; S: septal; TG: transgastric; RV: right ventricle; SAX: short axis; TEER: transcatheter edge-to-edge repair
It is essential to understand that interventionalists and imaging specialists approach the TV apparatus from different perspectives. During intraprocedural imaging, the TEE probe is behind the heart, generating TV images in the “valentine” position (Supplementary Figure 3A-Supplementary Figure 3C). However, movement of catheters during TTVI, spatial relationships of the TV with adjacent structures (particularly the IVC) and the direction of predominant annular dilation are better understood when labelling TV structures using the “attitudinal” nomenclature. CCT can also be used to demonstrate the anatomic relationships of the TV using a fluoroscopy-like display (Supplementary Figure 3D- Supplementary Figure 3F).
Clear and continuous communication and understanding between the interventional echocardiographist and TV interventionalist is key to procedural success. Speaking the same anatomical and directional language requires mutual knowledge of imaging, devices and the procedure, and can only be achieved by joint training of the TV transcatheter team. Standardisation of right chamber views using the “attitudinal” orientation and nomenclature (in which the displayed image and relationships of the TV leaflets with adjacent cardiac structures are identical irrespective of the imaging modality) provides the basis for a common language used by interventional and imaging specialists89.
Use of intracardiac echocardiography (ICE) is helpful in patients with insufficient imaging quality90,91,92 and will certainly increase once novel 4D catheters allowing 3D imaging and multiplanar reconstruction become broadly available93. This may reduce the need for general anesthesia in the future.
POST-PROCEDURAL MANAGEMENT
Since AF is highly prevalent in patients presenting with severe TR (about 70%), post-procedural anticoagulation with either warfarin/coumadin or non-vitamin K antagonist oral anticoagulants (NOAC) is usually required. Patients in sinus rhythm with no other indication for anticoagulation should use the same antiplatelet regimen as after transcatheter mitral valve repair (usually four weeks of aspirin plus clopidogrel, followed by aspirin daily). The optimal antithrombotic regimen following TTVR is unclear, although early experience indicates that a warfarin-/coumadin-based anticoagulation regimen (possibly combined with aspirin for at least one year) might be preferred.
The preprocedural diuretic regimen should be maintained for at least three months after the procedure to allow RV reverse remodelling. However, careful dose reduction may be required in TTVR patients who develop early post-procedural polyuria (usually within 24-48 hours) as a result of improved cardiac output and reduced venous congestion, or in those who develop symptomatic hypotension and/or renal failure over longer-term follow-up. Given the need for frequent modification of the post-procedural diuretic and HF medication regimen, most sites recommend initial outpatient follow-up at 1, 6, and 12 months, followed by annual review. Assessment may include blood tests for NT-proBNP, renal and liver function, transthoracic echocardiography, a six-minute walk-test and a quality-of-life questionnaire.
Although systematic evidence is lacking, early experience suggests that glifozines (sodium glucose cotransporter 2 [SGLT-2] inhibitors) may be of particular benefit in selected patients with right-sided HF following TV intervention due to their diuretic, nephro-protective and symptomatic effects.
Periprocedural and early post-procedural antibiotic prophylaxis should be used in all patients undergoing TTVI to prevent infective endocarditis. After discharge, established endocarditis prophylaxis guidelines should be followed, although a lower threshold for treatment might be appropriate due to an increased probability of bacteraemia in the venous circulation.
PROPOSED REQUIREMENTS FOR A HEART VALVE CENTRE WITH EXPERTISE IN TRICUSPID VALVE TREATMENT
A multidisciplinary Heart Team approach is recommended for the evaluation of patients with TV disease in a Heart Valve Centre with expertise in a broad spectrum of diagnostic and therapeutic solutions (Table 4),94. Essential requirements include an interventional cardiology team with broad expertise in heart valve interventions, experience in advanced multimodality TV imaging (including high quality CCT and CMR for specific indications) and easy access to cardiac surgery counselling. A cardiac surgery department on site with operator experience in TV surgery, as well as an intermediate and intensive care unit (or alternatively a dedicated structural and valve unit) with collaborative links with other cardiac services (particularly an HF team) are mandatory, when investigational and replacement systems are used.
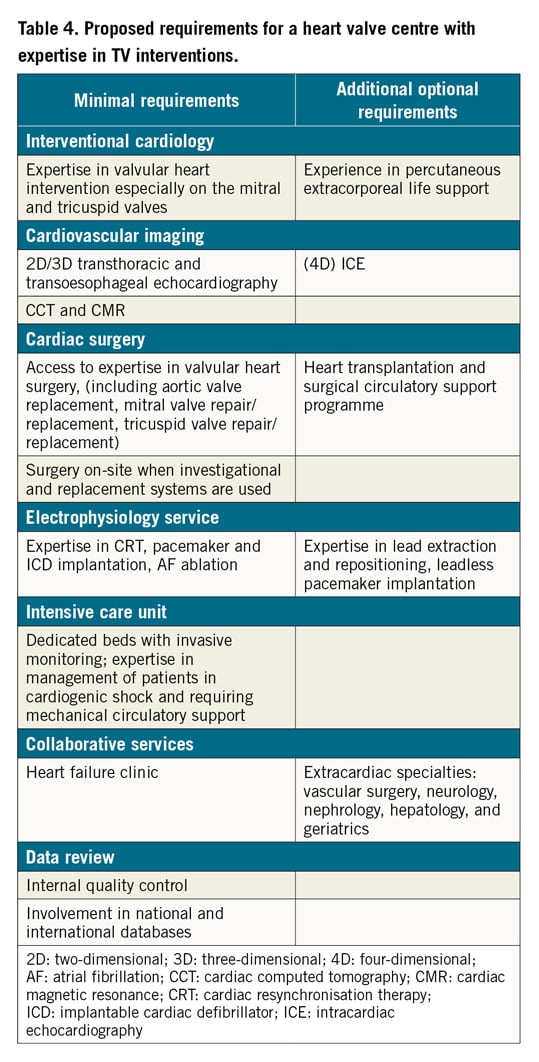
A Heart Valve Centre with expertise in TV treatment should build a referral and educational network with collaborating partners and offer easily accessible digital (imaging) data transfer solutions to enable remote consultation and case discussion. Finally, participation in multicentre studies assessing new treatment approaches for TR is of utmost importance given the need for greater understanding of the indications, timing and technical success of invasive TV treatments.
FUTURE OUTLOOK AND CHALLENGES
Although TTVI are rapidly emerging in response to an unmet clinical need, some important questions remain largely unanswered and require rapid resolution by means of large-scale registies and randomised studies:
– symptomatic and outcome benefits of STR correction compared to optimal medical treatment (TRILUMINATE, CLASP II TR, and TRISCEND II pivotal trials; TRI-FR; TRICI-HF)
– the appropriate timing of intervention in relation to clinical status, severity of TR, RV function and pulmonary artery pressure
– criteria for concomitant or staged TTVI in conjunction with interventions for aortic and/or mitral valve disease
– comparative safety and efficacy of established and emerging transcatheter treatment options
– clinical and echocardiographic indicators to avoid futile interventions.
As a first priority, the wide variability of practice in relation to the diagnosis, assessment and timely management of TV disease should be addressed and unified across Europe, and programmes to increase awareness amongst primary and secondary care providers promoted.
Alongside the established VARC95 and MVARC96 criteria, the definition of standardised endpoints and definitions will ensure homogenous event reporting, accurate adjudication, and appropriate comparisons of clinical research studies involving new devices and therapeutic strategies for the treatment of TV disease. Given that endpoints of future studies are likely to be based largely on quality of life measures, levels of physical activity and assessement of volume status, new innovative concepts including wearable technology97,98,99 and implantable HF monitoring devices will play an important role100. Since anatomical limitations, in particular large annulus size, still restrain eligibility, technological improvements are needed to address the needs of a broader population of patients. Advances in deep learning for the interpretation of echocardiographic, CCT and CMR images may further support standardisation and increase the accuracy of TR grading and assessement of RV function. These developments are set to accelerate rapidly in the next phase of the evolution of transcatheter valve interventions.
Appendix. Study collaborators.
Joao Cavalcante, MD, FASE, FACC, FSCCT, FSCMR; -Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis, MN, USA. Gilles Dreyfus, MD, FRCS, FESC; Institut Mutualiste Montsouris, Paris, France. Alison Duncan, MB BS, PhD, FRCP; The Royal Brompton Hospital, London, UK. Mara Gavazzoni, MD; Cardiovascular Imaging Unit, Department of Cardiovascular, Neural and Metabolic Sciences, Instituto Auxologico Italiano, IRCCS, San Luca Hospital, Milan, Italy. Julia Grapsa, MD, PhD, FACC; Guy's and St Thomas' NHS Trust, London, UK. Edwin Ho, MD, FRCPC; Azeem Latib, MD, BCh; Montefiore Medical Center, New York, New York, USA. Nicolo Piazza, MD, PhD; McGill University Health Center, Montreal, Quebec, Canada. Maurice Sarano, MD; Mayo Clinic, Rochester, MN, USA. Marta Sitges, MD, PhD; Cardiovascular Institute, Hospital Clínic de Barcelona, Bacelona, Spain & University of Barcelona, Barcelona, Spain. Martin Swaans, MD, PhD, FESC; St. Antonius Hospital, Nieuwegein, the Netherlands. Nina Wunderlich, MD, FACC; Cardiovascular Center Darmstadt, Darmstadt, Germany. Michel Zuber, MD, FESC; Cantonal Hospital, Aarau, Switzerland. Andrea Guidotti, MS; Simulands Ltd, Zurich, Switzerland. Scott Lim, MD; University of Virginia, -Charlottesville, VA, USA. Chaim Lotan, MD, FACC, FESC; Hadassah University Hospital, Jerusalem, Israel. Thomas Modine, MD; Cardiovascular Surgery Department, Cœur Poumon CHU de Lille, Lille, France. Luis Ortega-Paz, MD, PhD; Cardiovascular Institute, Hospital Clinic, IDIBAPS, Barcelona, Spain & Division of Cardiology, University of Florida College of Medicine, Jacksonville, FL, USA. Lars Sondergaard, MD; The Heart Centre, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. Hendrick Treede, MD; Department of Cardiac and Vascular Surgery, University Hospital Mainz, Johannes Gutenberg-University Mainz, Mainz, Germany. Rudiger Lange, MD; Department of Cardiovascular Surgery, German Heart Center Munich, Technische Universität München, Munich, Germany; Insure (Institute of Translational Cardiac Surgery), Department of Cardiovascular Surgery, German Heart Center Munich, Technische Universität München, Munich, Germany; DZHK (German Center for Cardiovascular Research) - Partner Site Munich Heart Alliance, Munich, Germany. Marianna Adamo, MD; Cardiac Catheterization Laboratory, ASST Spedali Civili di Brescia, Brescia, Italy. Martin Andreas, MD, MBA, PhD, MEBCTS; Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria. Stephan Baldus, MD; Department of Cardiology, University Heart Center, Cologne, Germany. Paolo Denti, MD; Progressive Cardiac Surgeon, San Raffaele University Hospital, Milan, Italy. Nicolas Dumonteil, MD; Clinique Pasteur, Toulouse, France; Didier Tchétché, MD; Clinique Pasteur, Toulouse, France; Rodrigo Estevez-Loureiro, MD, PhD, FESC; Interventional Cardiology Unit, University Hospital Alvaro Cunqueiro, Vigo, Spain. Luigi Fiocca, MD; Cardiovascular Department Papa Giovanni XXIII Hospital, Bergamo, Italy. Kai Friedrichs, MD; Herz- und Diabeteszentrum Nordrhein-Westfalen, Universitätsklinik der Ruhr-Universität Bochum, Bad Oeynhausen, Germany. Carmelo Grasso, MD; Cardio-Toraco-Vascular Department - CAST AOU Policlinico “G. Rodolico-S. Marco”, Catania, Italy. Nicole Karam, MD, PhD; Université de Paris, PARCC, INSERM, F-75015, European Hospital Georges Pompidou, Paris, France. Wolfgang Rottbauer, MD, PhD; Department of Internal Medicine II (Cardiology, Angiology, Pneumology, Intensive Care Unit), Ulm University Medical Center, Ulm, Germany. Volker Rudolph, MD; General and Interventional Cardiology, Heart & Diabetes Center NRW, University Hospital of the Ruhr University of Bochum, Bad Oeynhausen, Germany. Keti Vitanova, MD; Department of Cardiovascular Surgery, German Heart Center Munich, Technische Universität München, Munich, Germany and Insure (Institute of Translational Cardiac Surgery), Department of Cardiovascular Surgery, German Heart Center Munich, Technische Universität München, Munich, Germany. Jeroen Bax, MD, PhD, FESC, FACC; Department of Cardiology, Leiden University Medical Centre (LUMC), Leiden, the Netherlands. Michael Borger, MD, PhD; University Clinic of Cardiac Surgery, Leipzig Heart Center, Leipzig, Germany. Erwan Donal, MD, PhD; University of Rennes, CHU Rennes, Inserm, LTSI - UMR 1099, Rennes, France. Helge Möllmann, MD, PhD; Department of Cardiology, St. Johannes Hospital, Dortmund, Germany. Osama Soliman, MD, PhD, FACC, FESC; Department of Cardiology, Saolta Group, Galway University Hospital, Health Service Executive and National University of -Ireland Galway (NUIG), Galway, Ireland. Maurizio Taramasso, MD, PhD, HerzZentrum Hirslanden Zurich, Zurich, Switzerland. Holger Thiele, MD, FESC; Heart Center Leipzig at University of Leipzig, Department of Internal Medicine/Cardiology, Leipzig, Germany. Nicolas van Mieghem, MD, PhD; Department of Interventional Cardiology, Erasmus University Medical Centre, Rotterdam, the Netherlands. Stephan Windecker, MD; Department of Cardiology, Bern University Hospital, University of Bern, Bern, Switzerland. Luigi Badano, MD, PhD; Multimodality Cardiovascular Imaging Center, Department of Medicine and Surgery, University of Milano-Bicocca, Milan, Italy & Department of Cardiology, Metabolic and Neural Sciences, San Luca Hospital, Istituto Auxologico Italiano, IRCCCS, Milan, Italy. Manuel Barreiro, MD, PhD, FESC; Cardiovascular Imaging, Cardiology Department, University Hospital Alvaro Cunqueiro, Vigo, Spain. Lenard Conradi, MD; University Heart and Vascular Center Hamburg, Department of Cardiovascular Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. Neil Fam, MD MSc; St. Michael's Hospital, University of Toronto, Toronto, Canada. Xavier Freixa, MD, PhD; Hospital Clinic of Barcelona. University of Barcelona. IDIBAPS. Barcelona, Spain. Marco Metra, MD; University and Civil Hospitals of Brescia, Brescia, Italy. Gilbert Tang, MBA, MSc, MD; Department of Cardiovascular Surgery, Mount Sinai Health System, New York, NY, USA. Yan Topilsky, MD; Cardiovascular Imaging, Cardiology Department, Tel Aviv Medical Center, Tel Aviv, Israel. Alec Vahanian, MD; University of Paris, Paris, France. William Wijns, MD; The Lambe Institute for Translational Medicine and Curam, National University of Ireland Galway, Galway, Ireland.
Conflict of interest statement
F. Praz has received travel expenses from Abbott Vascular, Edwards Lifesciences, and Polares Medical. D. Muraru is part of the speaker’s bureau for GE Healthcare and received research equipment support from GE Vingmed. F. Kreidel has received speaker’s honoraria from Abbott Vascular. P. Lurtz has served as a consultant for Abbott Structural Heart, Edwards Lifesciences, and Medtronic. R. Hahn has received speaker fees from Boston Scientific, Baylis Medical, Edwards Lifescience and Medtronic, consulting fees for Abbott Structural, Edwards Lifesciences, Gore&Associates, Medtronic, Navigate, and Philips Healthcare, non-financial support from 3mensio, has equity with Navigate, and is the Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation. V. Delgado has received speaker fees from Abbott Vascular, Edwards Lifesciences, GE Healthcare, Novartis, MSD and Medtronic. M. Senni has received personal fees from Novartis during the conduct of the study and personal fees from Bayer, Abbott, Merck, AstraZeneca, Vifor Pharma and Boehringer outside the submitted work. R.S. von Bardeleben is a consultant for Abbott Vascular. G. Nickenig has received research funding from the Deutsche Forschungsgemeinschaft, the German Federal Ministry of Education and Research, the EU, Abbott, Edwards Lifesciences, Medtronic, and St. Jude Medical, and has received honoraria for lectures or advisory boards from Abbott, Edwards Lifesciences, Medtronic, and St. Jude Medical. J. Hausleiter has received speaker honoraria from Abbott Vascular and Edwards Lifesciences. A. Mangieri has received an institutional grant from Boston Scientific and is part of the advisory board of Boston -Scientific. J. Zamorano has received speaker honoraria from -Daichii Sankyo and Pfizer. B. Prendergast has received unrestricted educational and research grants from Edwards -Lifesciences and speaker/consultancy fees from Edwards Lifesciences, Abbott, and Anteris. F. Maisano reports grant and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, and Terumo, consulting fees, honoraria (personal and institutional) from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, has royalty income/IP rights from Edwards Lifesciences, and is a shareholder (including share options) of Cardiogard, Magenta, SwissVortex, Transseptalsolutions, Occlufit, 4Tech, and Perifect. M. Adamo has no conflicts of interest to declare. M. Andreas reports receiving grants and fees from Edwards Lifesciences, Abbott, and Medtronic. L. Badano reports receiving consulting fees from Edwards Lifesciences, Esaote, and Jansen, and speaker fees from GE Healthcare, Esaote, and Philips. S. Baldus reports receiving a research grant Abbott, and speaker fees from Abbott and Edwards. M. Barreiro reports receiving speaker fees Abbott Vascular, Philips Healthcare and Boston Scientific. J. Bax reports receiving speaker fees Edwards Lifesciences and Abbott. M. Borger reports that his hospital receives consulting fees and/or speakers' honoraria on his behalf from Edwards Lifesciences, Medtronic, Abbott and CryoLife. J. Cavalcante reports receiving consulting fees Edwards Lifesciences, Abbott Structural, Medtronic, Inc., Boston Scientific, and VDYNE. L. Conradi reports receiving consulting and speaker fees from Edwards -Lifesciences, Boston Scientific, Medtronic, and Abbott. P. Denti reports receiving a consulting fee from InnovHeart, and speaker fees from Abbott and Edwards. E. Donal reports receiving speaker fees from Abbott Vascular, Philips Healthcare and Boston -Scientific. G. Dreyfus reports being a consultant from Edwards -Lifesciences. N. Dumonteil reports receiving consulting and speaker fees from Abbott Vascular, Ancora Heart, Boston Scientific, Edwards -Lifesciences, and Medtronic. A. Duncan reports lectures/presentations for Abbott Vascular, Edwards Lifesciences, and -Boston Scientific. R. Estevez-Loureiro reports receiving speaker fees Abbott Vascular, Edwards Lifesciences, and Boston Scientific. N. Fam has no conflicts of interest to declare. L. Fiocca has no conflicts of interest to declare. X. Freixa reports receiving consulting and speaker fees from Abbott Vascular. K. Friedrichs reports receiving grants from Edwards Lifesciences. M. Gavazzoni reports receiving personal fees from Abbott Vascular. J. Grapsa has no conflicts of interest to declare. C. Grasso reports receiving fees from Abbott Medical, Boston Scientific, and Edwards -Lifesciences. A. Guidotti has no conflicts of interest to declare. E. Ho reports receiving support for attending meetings from Abbott, Edwards Lifesciences, and Medtronic. N. Karam reports receiving consulting fees from Abbott, Edwards Lifesciences, and Medtronic, and speaker fees from Edwards Lifesciences. R. Lange reports receiving consulting fees and royalties from Medtronic. A. Latib reports receiving consulting fees from Edwards -Lifesciences, Abbott, Medtronic, and Boston Scientific. S. Lim reports receiving grants from Abbott Vascular and Edwards -Lifesciences. C. Lotan has no conflicts of interest to declare. M. Metra reports receiving consulting fees from Amgen, -AstraZeneca, Servier, Vifor Pharma, and WindTree Therapeutics, and speaker fees from Abbott Vascular, and Edwards Therapeutics. T. Modine is a consultant for Boston Scientific, Medtronic, Edwards, MicroPort, GE, and Abbott, and has received a research support grant from Edwards. H. Möllmann reports receiving consulting and speaker fees from Abbott Vascular, Boston Scientific and Edwards Lifesciences. L. Ortega-Paz has no conflicts of interest to declare. N. Piazza reports receiving consulting fees from Medtronic, Peijia Medical, and Highlife. W. Rottbauer reports receiving funding for this ms from Edwards Lifesciences, and consulting fees from Edwards Lifesciences and Abbott. V. Rudolph reports receiving grants from Edwards Lifesciences and Abbott Vascular. M. Sarano has no conflicts of interest to declare. M. Sitges reports receiving grants from Abbott, Medtronic, and Edward Lifesciences, and consulting and speaker fees from Abbott, Medtronic, and Edwards Lifesciences. O. Soliman has no conflicts of interest to declare. L. Sondergaard reports receiving consulting and speaker fees from Abbott, Boston Scientific, Medtronic, and SMT. M. Swaans reports receiving consulting fees from -BioVentrix, Inc., and speakers fees from Abbott Vascular, Edwards -Lifesciences, and Philips Healthcare. G. Tang reports receiving consulting and speaker fees from Abbott Structural Heart and Medtronic. M. Taramasso repors receiving consultant fees from Abbott, Edwards Lifesciences, Shenqi Medical, Boston Scientific, 4tech, CoreMedic, Simulands, MTEx, and Occlufit, and speaker fees from Abbott, and Edwards Lifesciences. D. Tchétché reports receiving consulting fees Abbott, Boston, Edwards, and Medtronic. H. Thiele has no conflicts of interest to declare. Y. Topilsky reports receiving consulting fees from Edwards, and MitralTech, and speaker fees from Edwards, and Abbott. H. Treede has received research support from Abbott Vascular, Boston -Scientific, Edwards Lifesciences, JenaValve Technology, Medtronic, Tricares and -Abiomed. A. Vahanian reports consultancy for -CardioValve, and receiving consulting fees from Abbott Vascular, Edwards -Lifescience, and Medtronic. N. van Mieghem has received grants and personal fees from Abbott Vascular, Medtronic, Boston -Scientific, and PulseCath BV. K. Vitanova reports receiving speakers honoraria from Medtronic. S. Windecker reports receiving research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards -Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed. N. Wunderlich reports receiving consulting fees from Edwards Lifesciences, Holitstick Medical and Cardiac Implants, and speaker fees from Cardiac Dimension, GE, Philipps, Gore, Abbott, Edwards Lifesciences, Occlutech, and Alnylam. M. Zuber has received fees from Edwards Lifesciences, and Abbott.
Supplementary data
To read the full content of this article, please download the PDF.

