Abstract
When the incidence of tricuspid regurgitation is taken into account, along with its impact on functional status and long-term survival, tricuspid regurgitation is currently undertreated. Today, though transcatheter therapy of aortic, mitral- and pulmonic valve disease is well established, interventional treatment of tricuspid valve disease is still in its early stages. Currently, various promising devices are in different stages of development, but it is still too early to clarify which interventional approach in the future might result in functional and clinical success. Similarly, it is yet unclear which type of patient subpopulation will benefit from this type of treatment. Seen in the current context of the overall evolution in the adoption of catheter-based treatments for other types of structural heart disease, the need for and interest in effective interventional treatments for tricuspid regurgitation is growing.
Introduction
While transcatheter therapies for aortic, mitral- and pulmonic valve disease are well established, interventional treatment of tricuspid valve disease is still in its early stages of development. Currently, only percutaneous balloon valvuloplasty of tricuspid stenosis is supported by clinical evidence; however, this condition is rare and hardly encountered in western countries1. In contrast, clinically relevant tricuspid regurgitation (TR) by far outnumbers any other type of tricuspid valve disease. For TR, limited experimental data have been published and interventional treatment of TR has been reported in isolated compassionate human cases using investigational techniques. While various devices are currently in different stages of development, it is still unclear which interventional approach will eventually result in a functional and clinical success and in which type of patient subpopulation this will occur. Considering the incidence of TR and its implications on functional status and long-term survival, tricuspid regurgitation is currently undertreated. With the increasing adoption of catheter-based treatments of other types of structural heart disease, there is a growing interest and need for effective interventional treatments for tricuspid regurgitation as well. Therefore, multiple treatment approaches are under investigation and will be discussed below.
Clinical impact of tricuspid regurgitation (TR) in patients with structural heart disease
Tricuspid regurgitation (TR) is a common condition in patients with late stages of left heart valve or myocardial disease and has a significant impact on functional status and long-term survival. In the majority of patients, TR is not related to primary valve pathology, but rather is functional. Mild TR is frequent and does not affect patient prognosis; however, moderate or severe TR is recognised as an independent risk factor for poor outcomes2-5. The symptoms of right-sided heart failure are difficult to manage with medical therapy, and few patients are currently offered surgery for isolated tricuspid valve disease. Because isolated tricuspid surgery still carries an operative mortality of >20% in certain subpopulations, the procedure is frequently not performed in high-risk patients6.
Transcatheter repair of the tricuspid valve
The tricuspid annulus has a greater variability and offers less resistance for prosthetic valve fixation than the mitral annulus or even the aortic annulus. Due to a lower proportion of fibrous tissue, size and flexibility of the annulus varies considerably under different pre- and after-load conditions, and the development of severe TR is associated with a further loss of anatomic landmarks. While edge-to-edge repair or coronary sinus-related approaches are not feasible on the tricuspid valve, other percutaneous approaches developed for mitral valve repair could be suitable for transfer to the tricuspid valve.
For example, in a procedure analogous to one for the mitral valve, percutaneous tricuspid annular plication has successfully been applied on the tricuspid valve. The Mitralign® Percutaneous Annuloplasty System (Mitralign, Tewksbury, MA, USA) was used on the tricuspid valve in a compassionate human case to achieve circumferential annulus reduction7. With the Mitralign two-armed Bident catheter2-4, pledget-enforced anchors were placed through the tricuspid annulus and plicated together, resulting in annuloplasty and bicuspidisation with reduction of TR.
The advantage of this concept for treatment of TR is that it can be performed irrespective of the annulus size and adjacent anatomic structures. Also, no implant is left behind. Further, a “partial” correction of TR is possible to avoid the haemodynamic burden8 of acute complete correction of TR to the right ventricle. Whether this technique achieves durable results will have to be investigated in clinical studies. A potential limitation results from the complex steerability of the device in the right ventricle, and procedural success depends heavily on non-invasive imaging quality.
Annuloplasty might also be performed by means of percutaneous ring implantation.
One of the investigational devices currently under development specifically for tricuspid valve repair is the Millipede annular ring (Millipede, LLC, Ann Arbor, MI, USA). This device mimics surgical annuloplasty by transcatheter implantation of an expandable and contractable ring that uses a novel attachment technique with multiple small barbed anchors to secure it in place. Prior to ring fixation, the annulus is expanded by a dilator to the size and shape of the ring9. The ring is then anchored to the annulus and contracts to a predefined size, resulting in size reduction of the annulus.
The TriCinch System™ (4Tech Cardio Ltd, Galway, Ireland) is another technology aimed at reducing the degree of TR. By transcatheter placement of a screwed anchor in the septal portion of the TV annulus, tension is applied reducing the septolateral diameter of the annulus and subsequently reducing the degree of regurgitation as well. Tension is maintained through a wire connecting the screw to a self-expandable stent anchored in the inferior vena cava. The device is currently investigated in a phase 1 clinical trial.
Transcatheter replacement of the tricuspid valve
Although transcatheter valve repair may be a preferred treatment, it is uncertain whether any of the above concepts can be successfully transferred to routine clinical practice. Any repair procedure has to yield predictable and reproducible results in the hands of a wide range of interventional cardiologists to be established in clinical practice. Therefore, percutaneous valve implantation could be a superior alternative to percutaneous repair techniques. From the interventional perspective, there are two basic approaches for percutaneous tricuspid valve replacement, depending on the implantation position of the prosthetic valve – orthotopic versus heterotopic valve replacement.
In orthotopic valve replacement, a prosthetic valve is deployed at the level of the TV annulus between the right ventricle and right atrium. In human patients with a failing bioprothesis or surgical rings, orthotopic tricuspid valve replacement has been applied with good haemodynamic and clinical results as a valve-in-valve or a valve-in-ring procedure, using either the balloon-expandable Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) or the Melody® valve10-13 (Medtronic, Minneapolis, MN, USA). In a retrospective multicentre registry, Godart et al summarise the data of 71 patients undergoing valve-in-valve tricuspid replacement after bioprosthetic surgical device failure. The authors reported a high procedural success rate and functional improvement in nearly all cases. Device migration into the right ventricle was observed as a periprocedural complication in two cases8. Although longer follow-up data are still missing, the percutaneous approach can be considered as a preferred treatment in those patients requiring reintervention on the tricuspid valve after previous surgical valve or ring implantation.
In contrast, percutaneous orthotopic valve implantation in the native tricuspid annulus has never been applied in human patients. This approach was initially investigated by Boudjemline et al in an animal model by means of implanting a double-disc nitinol stent with a bioprosthetic valve into the tricuspid annulus14. Particularly in functional TR, annulus dilatation may reach >70 mm and is associated with a loss of anatomic landmarks between the right ventricle (RV) and right atrium (RA). A device intended for orthotopic TV replacement would require unique solutions for stent and catheter design as well as tissue valve engineering (e.g., a 70 mm tissue valve would require a profile of >40 mm to avoid prolapsing into the RA).
A preferable alternative to the orthotopic approach is heterotopic valve replacement.
Caval valve implantation (CAVI) involves the implantation of stent valves into the inferior and superior vena cava. The haemodynamic effects of this approach were demonstrated in animal models with acute TR, where the implantation of caval valves resulted in an immediate recovery of cardiac output and a significant reduction of caval regurgitation (human data Figure 1)15. Meanwhile, this concept has been applied for compassionate treatment in human patients using either custom-made self-expandable valves or the balloon-expandable Edwards SAPIEN XT valve16,17 (Edwards Lifesciences). CAVI is technically simpler than the above-mentioned repair procedures. Another potential advantage is that this approach does not interfere with any pre-existing transtricuspid pacemaker or defibrillator leads crossing the tricuspid annulus, which may present a limitation for orthotopic procedures on the tricuspid valve.
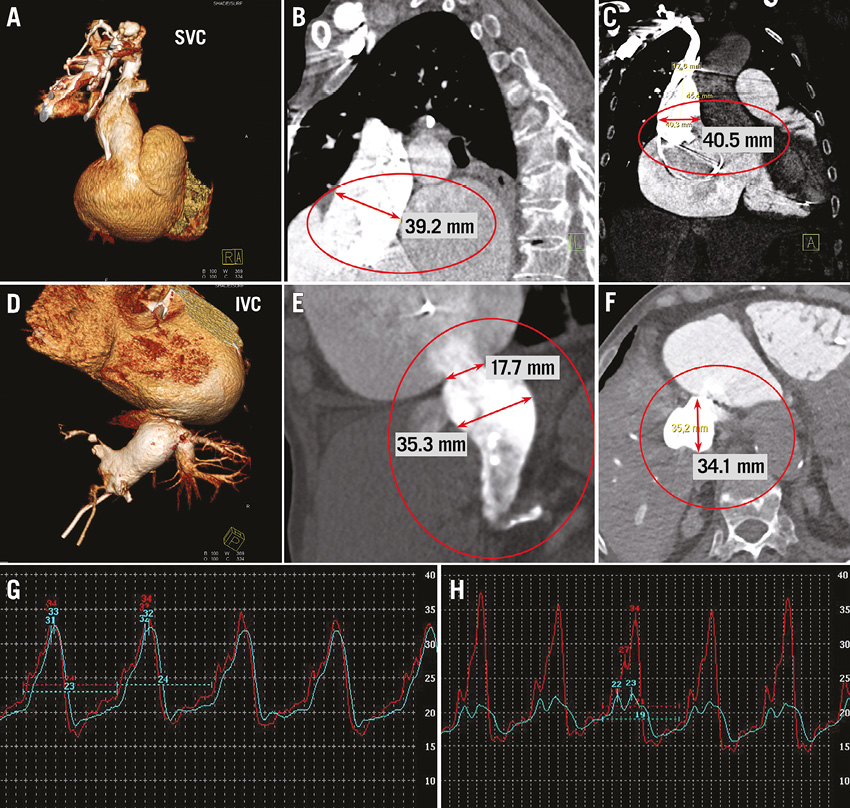
Figure 1. Transcatheter treatment of tricuspid regurgitation: anatomic suitability for caval valve implantation. CT angiography with 3D volume rendering demonstrates a massive enlargement of the superior vena cava (A-C) and inferior vena cava (D-F). Pressure tracings before (G) and after (H) caval valve implantation (blue tracing=inferior vena cava; red tracing=right atrium). Invasive pressure measurement confirms a reduction of the v-wave and mean pressure in inferior vena cava pressure from 32 mmHg to 23 mmHg and 24 mmHg to 19 mmHg, respectively.
Nevertheless, limitations apply as well to heterotopic procedures. From a haemodynamic perspective, CAVI does not address TR itself but the regurgitation of blood into the caval veins. As this condition is present only in a subgroup of patients with severe, often long-standing TR and RV enlargement, haemodynamic proof of caval regurgitation is essential before valve implantation. Also, it is yet unclear whether this subpopulation benefits from an interventional procedure. The persistence of right atrial volume overload and the ventricularisation of the right atrium are potential limitations of the procedure; its long-term impact on right atrial and ventricular function is currently unknown.
Under the condition of severe TR, there are considerable variations in the anatomic diameters of the superior vena cava and inferior vena cava, which frequently exceed the suitable range for implantation of current, commercially available devices (Figure 1 A-F). Regarding the Edwards SAPIEN XT valve, this drawback has been partially compensated for by pre-stenting the caval veins prior to valve deployment for downsizing and improved valve anchoring. In this position, self-expandable devices specifically designed for caval valve implantation are likely to be a superior alternative as they do not require a pre-stenting of the landing zone. Self-expandable valves for commercial use are currently under development18.
Conflict of interest statement
The authors have no conflicts of interest to declare.

