
Introduction
Although the tricuspid valve (TV) was initially considered the “forgotten valve”, the management of TV pathologies has re-emerged in recent years1. In the USA, moderate to severe tricuspid regurgitation (TR) affects nearly two million people and increases mortality, prolongs hospitalisations, and increases rates of rehospitalisation2,3,4. Furthermore, patients with TR can have significant clinical symptoms and decreased quality of life (QoL)5. Despite nearly 200,000 new diagnoses of TR per year in the USA, only 8,000 TV operations are performed annually6, highlighting the need for additional treatment options for TR patients.
Surgical intervention remains high-risk in patients with severe TR and progressive heart disease7. Patients who commonly develop TR after left-sided cardiac surgery are also a high-risk cohort. In recent years, transcatheter strategies have emerged as a minimally invasive approach for these high-risk patients with TR. As preclinical and clinical data continue to accumulate8,9, transcatheter repair of TR appears to be a promising strategy. In this perspectives article, we choose to highlight the five Ws (Who, What, When, Where, and Why) of transcatheter repair of functional TR. We discuss the current stages of transcatheter repair of TR and the next steps required for further translation.
Who: who benefits from transcatheter repair of TR?
Experience of transcatheter repair of TR, which has emerged only in recent years, has mainly been in preclinical and compassionate use settings8. Only in the last several years have transcatheter approaches transitioned into early clinical trials, evaluating feasibility, efficacy, and safety in patients with high-grade TR. In these trials, the feasibility and safety of transcatheter repair of TR have largely been proved successful8,9. However, clinical efficacy has varied widely depending on the transcatheter approach. Although evaluation of anatomic approaches and earlier transcatheter implementation strategies is required, we anticipate a growing need for transcatheter repair in select patients with TR. In recent years, the cardiac patient population with severe TR has become more complex - patients are older, have had prior cardiac surgery, have significant cardiac and non-cardiac comorbidities (atrial fibrillation, chronic obstructive pulmonary disease [COPD], kidney disease), have sequelae of advanced disease (ascites, peripheral oedema, etc.), are in New York Heart Association (NYHA) functional Class III/IV, are diuretic dependent, have had multiple previous admissions for right ventricular (RV) failure, and have a high effective regurgitant orifice area (EROA)10. As such, the targeted population represents patients with TR who have a prohibitive or high operative risk. This includes patients with TR and severe comorbidities or patients who remain high-risk after prior left-sided cardiac surgery because of the risks of reoperation. As these patients are a high-risk cohort for surgery, they should be an immediate target for transcatheter repair of TR.
We also suspect that, with increasing efficacy and safety, asymptomatic patients with severe TR and patients with mild or moderate TR with annular dilation without an indication for cardiac surgery may also be candidates for transcatheter repair in the near future. This is logical, especially if they are already undergoing a transcatheter aortic or mitral procedure. Earlier implementation would prevent the development of irreversible tricuspid annular (TA) dilatation, RV dilatation, and leaflet tethering, which play key roles in the pathophysiology of TR.
What: what transcatheter technology should be used for TR repair?
There are several anatomic approaches through which transcatheter therapies achieve TR repair. While some strategies replicate highly effective surgical techniques, including suture- and ring-based annuloplasty, others replicate less effective surgical techniques (edge-to-edge repair, leaflet augmentation, etc.). At present, there are three major categories of device that replicate surgical techniques, including coaptation, suture-based annuloplasty, and ring-based annuloplasty.
COAPTATION DEVICES
Three major devices exist, namely the MitraClip® (Abbot Vascular, Santa Clara, CA, USA), PASCAL (Edwards Lifesciences, Irvine, CA, USA), and FORMA (Edwards Lifesciences) systems. The MitraClip device, which implants clips on the TV leaflets to reduce regurgitant flow, has become a first-line approach for TR with over 2,000 patients treated worldwide (Figure 1A). The Trial to Evaluate Treatment with Abbott Transcatheter Clip Repair System in Patients with Moderate or Greater Tricuspid Regurgitation (TRILUMINATE) enrolled 85 patients with 90% of patients having at least one-grade improvement in TR11. With the new TriClip delivery system (Abbott Vascular), the TRILUMINATE Pivotal Trial (NCT03904147) has already started enrolment for patients with severe TR. The PASCAL system is another promising device that has broad paddles, independent leaflet grasping and a central spacer that allows leaflets to be captured while at the same time acting as a spacer to fill the regurgitant orifice area (Figure 1B). Compassionate-use programmes for patients with severe TR have shown promise, an early feasibility study has recently been completed and a larger pivotal study has been planned11. Although the programme has recently been stopped, the FORMA Repair System uses an expandable balloon spacer to decrease regurgitant area in the tricuspid annulus (TA), providing a new coaptation surface for leaflets. Overall, although these devices have been used in early feasibility and safety trials, most patients have continued to have moderate TR post intervention12,13. Although considered an overall clinical success given baseline patient characteristics14, further work is required to achieve even better efficacy similar to surgical outcomes.
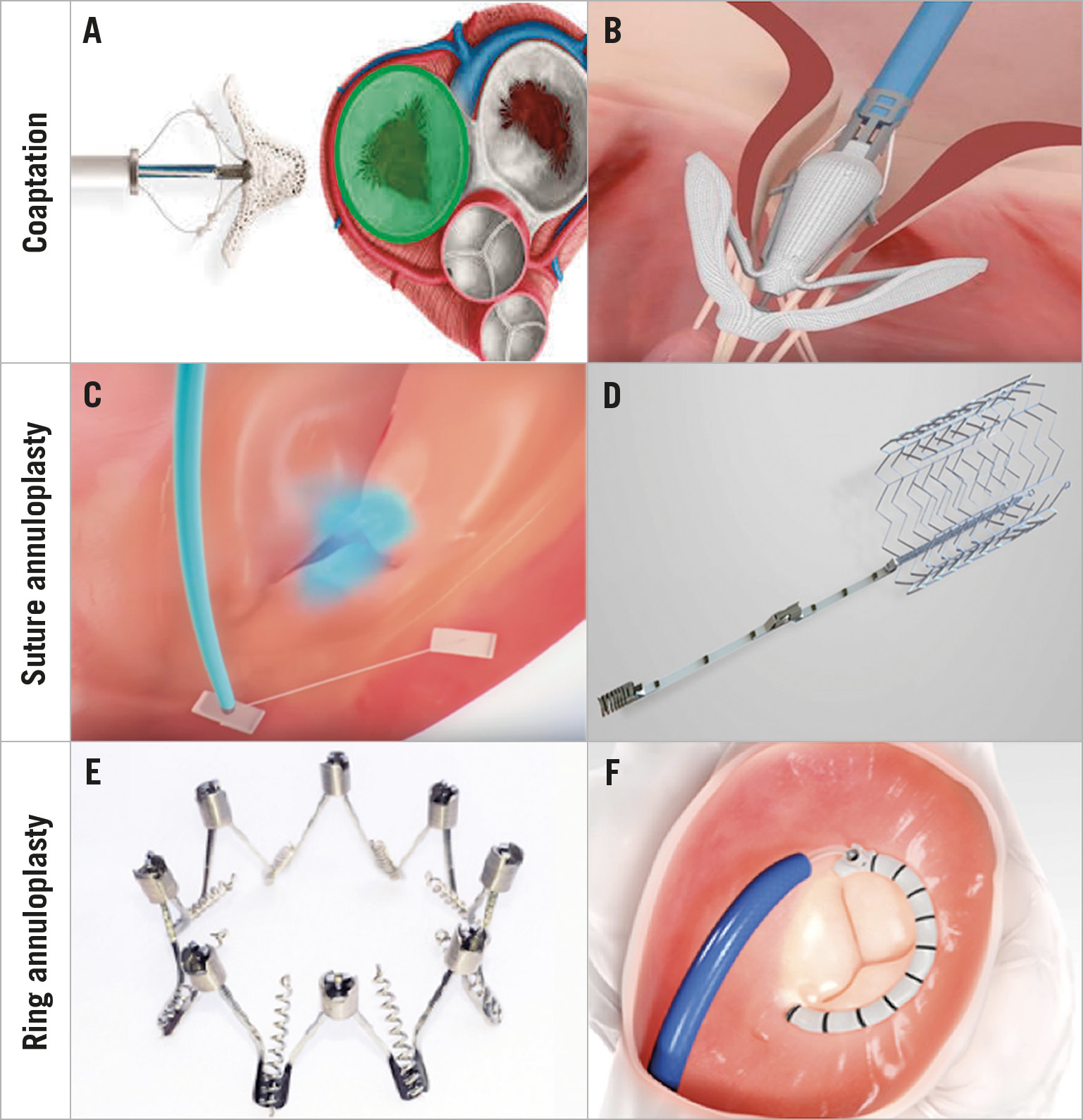
Figure 1. Transcatheter approaches. Several anatomic techniques replicating surgical strategies exist including coaptation, suture annuloplasty, and ring annuloplasty. A) The MitraClip System. B) PASCAL System. C) TriAlign System. D) TriCinch System. E) Millipede IRIS System. F) Cardioband Tricuspid Repair System.
SUTURE-BASED ANNULOPLASTY THERAPIES
The Trialign™ device (Mitralign, Tewksbury, MA, USA), which no longer exists, replicates the modified Kay bicuspidisation suture annuloplasty by using a plication device to cinch pledgets to eliminate the posterior tricuspid leaflet (Figure 1C). Similarly, the TriCinch System™ (4Tech Cardio, Galway, Ireland) replicates the Kay annuloplasty technique by reducing the septolateral diameter of the TA by tightening the anteroposterior commissure via an extracardiac anchor (Figure 1D). Early studies of these devices have demonstrated some degree of TR grade reduction, but moderate to severe TR often remains in these patients15,16. As such, further work is required to achieve results similar to surgery, which includes no to minimal residual TR following intervention.
RING-BASED ANNULOPLASTY TECHNIQUES
The Millipede IRIS System (Boston Scientific, Marlborough, MA, USA) involves a completely adjustable, semi-rigid annuloplasty ring that mimics the surgical gold standard of a rigid, ring-based annuloplasty (Figure 1E). The device has been surgically implanted using a transcatheter system in two patients with TR17 and both are greater than two years from intervention with no evidence of recurrent TR. The Cardioband Tricuspid Repair System (Edwards Lifesciences), which is the first device commercially approved for TR in Europe, enables a partial, cinchable band to be placed at the TA, reducing the anteroposterior and septolateral TA dimensions (Figure 1F). The TrIcuspid Regurgitation RePAIr with CaRdioband Transcatheter System (TRI-REPAIR) trial has already demonstrated efficacy in more than 30 patients14. Given such promise, additional clinical trials are underway.
Overall, transcatheter approaches for TR repair have been primarily utilised in compassionate cases and with limited numbers of high-risk patients (severe, massive, or torrential TR) in early trials. Although these patients had TR grade reduction and improvement in QoL, most continued to have moderate to severe TR. Although acceptable for compassionate use or in patients without other therapeutic options, a gap remains in achieving results similar to surgery, which includes no to minimal residual TR. It is worth highlighting, however, that these patients often have a different phenotype compared to surgical patients, as many are not candidates for surgery. These patients are often highly complex with significant TR disease progression, including more advanced TV and RV remodelling18. As these patient cohorts are clearly different, this may explain why single transcatheter devices have been less effective than surgery. Despite this, transcatheter repair of TR should still aim to replicate surgical outcomes.
Further work is also required to determine the optimal transcatheter approach for the repair of TR. We suspect that this may include a combination of several transcatheter approaches or other innovative strategies. We also suspect that replicating well-established surgical techniques, including rigid, ring-based annuloplasty which addresses TA dilatation and severely diseased leaflets, and valve replacement which addresses leaflets not amenable to repair, will be successful strategies in the transcatheter repair of TR.
When: when should this technology be used in the TR disease process?
At present, most of the transcatheter technology has been evaluated late in the progression of TR, involving patients with severe, massive, or torrential TR. For compassionate use, this may be appropriate. However, it fails to target the early window leading to irreversible pathologic changes resulting from TR. As surgical ring-based annuloplasty is administered for early TA dilation treatment with or without TR during left-sided valve surgery, we feel that transcatheter therapies for TR should attempt to mimic this implementation strategy. Furthermore, as the RV and TA exhibit cardioplasticity following TR regression19, a more aggressive approach should be implemented. As early feasibility and safety have now been demonstrated14, we suspect that the next step would be applying transcatheter repair safely in patients with mild to moderate TR with annular dilatation. This may allow reversal of RV alterations, involving TA diameter and TV coverage. Until randomised studies showing that treatment of functional TR is superior to medical management are completed, we suspect that this will probably occur in patients undergoing concurrent transcatheter therapy for mitral and aortic valve pathology. However, further studies are required to determine which patients will benefit from early transcatheter repair of TR. Hopefully, transcatheter therapy will let us approach the “earlier” TR patients.
Where: where should a patient receive transcatheter repair of TR?
As transcatheter therapy for TR repair has become an evolving field in recent years, we recommend that patients receive this therapy at select academic tertiary medical centres. These centres are generally involved in cutting-edge transcatheter research in association with industry. These institutions are filled with experienced interventional cardiologists, cardiac surgeons, and interventional radiologists and imagers/echocardiographers. This dedicated team is responsible not only for appropriate patient and device selection, but also for real-time imaging during device placement. As such, this experienced team structure provides patients with the highest clinical efficacy and safety following transcatheter repair of TR.
Why: why should transcatheter repair for TR be pursued?
Patients can be affected by TR in several ways. Patients often have decreased cardiac output, resulting in fatigue and decreased exercise tolerance, and right-sided heart failure causing ascites, oedema, and decreased appetite, all of which contribute to decreased QoL2,3,4. More importantly, moderate to severe TR has been shown to increase mortality independently from pulmonary artery systolic pressure, left ventricular ejection fraction, inferior vena cava size, and RV size and function2,3,4. In recent years, additional studies have confirmed the correlation between TR severity and increased mortality2,3,4. Furthermore, moderate and severe TR have been shown to be linked to prolonged hospitalisation and higher rates of rehospitalisation2,3,4. Recent studies have also demonstrated that isolated TV surgery is rarely performed, and that in-hospital mortality has remained high and unchanged over the last 10 years7. Furthermore, the majority of patients with severe TR evaluated in tertiary centres are not considered to be surgical candidates18. As such, these patients should undergo transcatheter repair of TR to improve symptoms and decrease the morbidity and mortality of TR. As surgery remains high-risk in select patient cohorts, transcatheter repair of TR provides a lower-risk and less invasive treatment option. We envision that targeting these patients earlier in their TR disease progression will be a priority in the coming years.
Conclusion
In conclusion, transcatheter therapies remain a promising avenue for patients who are high-risk for surgical repair of TR. Although numerous transcatheter repair strategies exist, we suspect that a combination of existing transcatheter approaches or those replicating successful surgical techniques, including ring-based annuloplasty and valve replacement, will be the most efficacious. Overall, further work remains to be done in the translation of transcatheter therapies for TR, which includes achieving no to minimal residual TR after intervention. We suspect that earlier implementation of transcatheter repair of TR, which will minimise disease progression, is the next step in the translation of this technology.
Conflict of interest statement
S.F. Bolling is a consultant to Boston Scientific. A. Latib is a consultant to Medtronic, Abbott, and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

