
Abstract
Severe tricuspid regurgitation (TR) has long been neglected despite its well-known association with mortality. While surgical mortality rates remain high in isolated tricuspid valve surgery, interventional TR repair is rapidly evolving as an alternative to cardiac surgery in selected patients at high surgical risk. Currently, interventional edge-to-edge repair is the most frequently applied technique for TR repair even though a device has not been developed for this particular indication. Due to the inherent differences in tricuspid and mitral valve anatomy and pathology, percutaneous repair of the tricuspid valve is challenging due to a variety of factors including the complexity and variability of tricuspid valve anatomy, echocardiographic visibility of the valve leaflets, and device steering to the tricuspid valve. Furthermore, it remains to be clarified which patients are suitable for a percutaneous tricuspid repair and which features predict a successful procedure. On the basis of the available experience, we describe criteria for patient selection including morphological valve features, a standardised process for echocardiographic screening, and a strategy for clip placement. These criteria will help to achieve standardisation of valve assessment and the procedural approach, and to develop interventional tricuspid valve repair further, using either currently available devices or dedicated tricuspid edge-to-edge repair devices in the future. In summary, this manuscript will provide guidance for patient selection and echocardiographic screening when considering edge-to-edge repair for severe TR.
Abbreviations
HFpEF: heart failure with preserved ejection fraction
HFrEF: heart failure with reduced ejection fraction
MR: mitral regurgitation
MV: mitral valve
NYHA: New York Heart Association
RA: right atrium
RV: right ventricle
TAVR: transcatheter aortic valve replacement
TEE: transoesophageal echocardiography
TR: tricuspid regurgitation
TTE: transthoracic echocardiography
TV: tricuspid valve
Introduction
Severe tricuspid regurgitation (TR) has been shown to be associated with significant morbidity and mortality and leads to peripheral oedema, ascites, reduced cardiac output, and heart failure1-5. Current guidelines recommend surgical tricuspid valve repair in patients with severe symptomatic TR. In addition, tricuspid valve repair should be considered in patients with mild to moderate TR due to annulus dilatation undergoing left-sided valve surgery6,7. However, due to comorbidities, the majority of patients are ineligible for surgical tricuspid valve repair.
The edge-to-edge repair technique has been established as an effective and durable alternative treatment option for patients with mitral regurgitation (MR) at prohibitive risk for cardiac surgery8-10. A significant proportion of patients with symptomatic MR also suffer from right-sided heart failure complicated by clinically relevant TR, which is usually secondary in origin. In approximately 50% of patients, the TR persists after interventional MR repair, while some reduction in TR severity is seen within six months after MR repair in the remaining 50% of patients11-13. Interestingly, patients with combined MR and TR have a higher early and late mortality despite successful MR repair when compared to patients with successful repair of an isolated MR14.
Although various concepts for interventional tricuspid valve repair are currently under development and clinical testing, there is no dedicated interventional tricuspid valve repair system available15. Recently, the edge-to-edge repair technique has been successfully applied for the treatment of severe TR in selected patients with isolated TR or combined MR and TR16-18. Similar to the edge-to-edge repair of the mitral valve, the TR is treated by connecting two leaflets by clip placement, most frequently between the anterior and septal as well as the posterior and septal leaflets. A broader use of this technique has been hindered for several reasons: (1) a higher anatomic complexity and variability of the tricuspid valve compared to the mitral valve, (2) lack of criteria for valve morphology addressing the suitability for edge-to-edge repair, (3) lack of standardised echocardiographic imaging protocols for patient screening and procedural guidance, (4) difficulties in device steering in the right atrium to accomplish perpendicularity with the tricuspid plane for leaflet grasping, and (5) lack of routinely successful strategies for clip placement to achieve optimal TR reduction.
Based on early and limited experience, the rationale of this manuscript is to provide guidance for patient selection and echocardiographic screening when planning tricuspid edge-to-edge repair. The recommendations are based on the published as well as unpublished experience with the currently available edge-to-edge repair system, which has been developed for the treatment of MR but applied for tricuspid repair on an off-label basis to over 400 patients worldwide. As dedicated tricuspid edge-to-edge repair systems are currently in development, most of the current considerations will also be valid and transferable to dedicated next-generation tricuspid edge-to-edge repair systems.
TR AETIOLOGY, TR ASSESSMENT AND TREATMENT STRATEGY
In the majority of patients, TR is of secondary (functional) origin, which typically includes RV and/or tricuspid annular dilatation, pulmonary hypertension, and regional or global RV systolic dysfunction. Secondary TR commonly develops by annular dilatation, which is mainly caused by a progressive outward remodelling of the free wall of the right ventricle and/or right atrium, which leads to papillary muscle displacement and tethering of the tricuspid leaflets19. Significant TR itself leads to further RV and RA dilation and dysfunction, enhancing tricuspid annular dilatation and tethering, and worsening TR. Remodelling of the annulus mainly occurs at the site of the anterior and the posterior tricuspid leaflets. Accordingly, it can be hypothesised that a promising edge-to-edge repair strategy appears to be to counteract the outward pulling forces by clip placement in the anteroseptal and posteroseptal coaptation lines. This will not only improve leaflet coaptation but also provide inward pulling of the dilated tricuspid annulus and adjacent free right ventricular wall in addition to a potential shift of the septum towards the RV (Figure 1). If feasible, clips may be placed on both coaptation lines as centrally as possible to maximise the inward pulling effect. Clip placement on the anteroposterior coaptation line is expected to be less effective for inward remodelling of the tricuspid annulus and may even further impair the coaptation with the septal leaflet by bringing the coaptation line of the anterior and posterior leaflet under tension. Therefore, depending on leaflet and valve size, dual clip placement in the anteroseptal and posteroseptal coaptation lines appears to be most effective for treatment of secondary TR. This approach will maintain a sufficient valve opening area with multiple orifices without risking relevant tricuspid valve stenosis. Similar conclusions have been reached in an ex vivo model for interventional TR repair20. At the current stage, however, it is unknown what value of trans-tricuspid valve gradients can be accepted for interventional TR procedures before symptomatic tricuspid valve stenosis occurs. The available experience supports the notion that inflow mean gradients of ≤3 mmHg are clinically acceptable in the low-pressure conditions of the right heart.
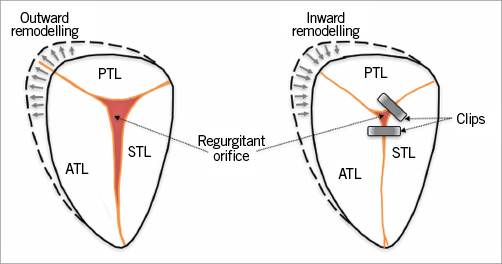
Figure 1. Progressive dilatation of the tricuspid annulus with outward remodelling leading to secondary TR (left). A strategy of clip placement in the anteroseptal and posteroseptal coaptation lines will result in an inward remodelling, which will improve leaflet coaptation. ATL: anterior tricuspid leaflet; PTL: posterior tricuspid leaflet; STL: septal tricuspid leaflet
Primary or degenerative TR is observed less often, but the percutaneous edge-to-edge repair technique has been applied successfully in selected patients for treating severe primary TR21. Although the available experience with primary TR is sparse, the EVEREST criteria may also be applied for the identification of a prolapse of acceptable width and height to allow interventional treatment.
Assessment and grading of TR severity has been described in detail elsewhere22,23. Grading the severity of TR is in principle similar to MR, but standards for judging TR severity are less robust than for MR. Although the current European and American guidelines distinguish only between mild, moderate and severe TR, a fourth grade for a massive, torrential TR is often added in clinical practice. This fourth grade describes massive TR in patients in whom the tricuspid valve has essentially lost its valve function with progressive equalisation of RV and RA pressures. Furthermore, a new grading scheme for TR comprising five grades has recently been proposed to extend the current three grades scheme, which does not provide adequate differentiation at the upper end of the severity scale, introducing grades of “severe”, “massive”, and “torrential” TR24. It must be remembered that the echocardiographic TR assessment only provides a snapshot of the TR at a single time point, not taking into account multiple factors including intravascular volume status, blood pressure, heart rate and arrhythmia (e.g., atrial fibrillation) known to influence the severity of TR in a given patient. The use of sedatives, narcotics and catecholamines during transoesophageal echocardiographic (TEE) screening or intraprocedurally may further affect the severity of TR at a certain point in time. Accordingly, the timing of echocardiographic TR assessment for percutaneous valve repair needs to respect the intravascular volume status and the medical heart failure optimisation due to the dynamic changes of TR and valve dimensions (including coaptation defect size).
ECHOCARDIOGRAPHIC SCREENING FOR EDGE-TO-EDGE TRICUSPID REPAIR
Two-dimensional (2D) TEE imaging is a mandatory investigation to assess valve suitability for TR edge-to-edge repair, if the preceding transthoracic echocardiography (TTE) study did not reveal a clear contraindication to percutaneous tricuspid repair. Simultaneous 2D-TEE biplane imaging, derived from a 3D-TEE matrix probe, is extremely helpful to identify the tricuspid leaflets and their function, with the primary plane displaying an RV inflow-outflow view while the secondary perpendicular plane is moved along the commissural length of the septal leaflet to visualise the coaptation of the septal-anterior (i.e., adjacent to the aorta) and septal-posterior commissure (i.e., towards the posterior wall). Accordingly, this simultaneous 2D-TEE biplane imaging should be the preferred technique for tricuspid valve visualisation using not only the mid-oesophageal imaging plane, but also deep oesophageal views which brings the tricuspid valve closer to the TEE probe. In case of suboptimal TEE images, 2D-TTE may be used as an additive and supportive approach in selected patients, but it cannot replace 2D-TEE due to usually poor visualisation of the posteroseptal and anteroposterior commissures. When using 2D-TTE imaging during the grasping process, the use of simultaneous fluoroscopy should be limited to avoid unnecessary radiation exposure of the echocardiographer holding the echo transducer at the thorax. In contrast to the edge-to-edge repair of the mitral valve, anatomic full-volume tricuspid 3D-TEE is only rarely of sufficient quality to provide additional information except for approximate clip location and orientation. This is due to the tricuspid valve being more distant to the probe, the presence of various interfering anatomical structures such as the aortic annulus, calcifications or left heart prosthetic valves, and the only faint echogenicity of the thin leaflets of the tricuspid valve.
In the following section, a standardised echocardiographic protocol is described, which will help to guide patient selection. It is important to perform the most important screening views also in a supine patient position, because the intrathoracic position of the heart and subsequently available echocardiographic windows may change significantly if a patient is studied in the commonly used left lateral patient position compared to the supine position used during the interventional procedure.
1) Transgastric basal short-axis view (Figure 2, Figure 3, Moving image 1, Moving image 2):
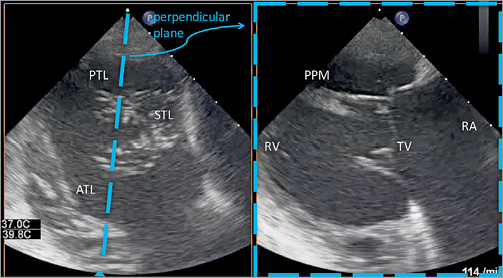
Figure 2. Biplane transgastric view of the tricuspid valve during mid systole by TEE. ATL: anterior tricuspid leaflet; PPM: posterior papillary muscle; PTL: posterior tricuspid leaflet; RA: right atrium; RV: right ventricle; STL: septal tricuspid leaflet; TV: tricuspid valve
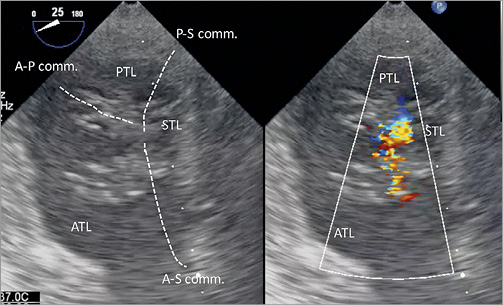
Figure 3. Simultaneous visualisation of the transgastric short-axis view of the tricuspid valve without (left) and with (right) colour Doppler. A-P comm: anteroposterior commissure; A-S comm: anteroseptal commissure; ATL: anterior tricuspid leaflet; P-S comm.: posteroseptal commissure; PTL: posterior tricuspid leaflet; STL: septal tricuspid leaflet
– commonly obtained between 25-50°
– only 2D view that allows a simultaneous visualisation of all three leaflets and commissures
– helpful to quantify the tricuspid annulus size and to assess leaflet motion and coaptation gaps
– location, vena contracta width and vena contracta area of the regurgitant jet can be visualised and severity of TR can be assessed by adding colour Doppler flow (Figure 3, Moving image 2)
– for steering the clip intraprocedurally to the desired position within the annular plane and to rotate the clip device to achieve perpendicularity with the respective leaflet coaptation line.
2) Midoesophageal TEE (Figure 4, Figure 5, Moving image 3, Moving image 4):
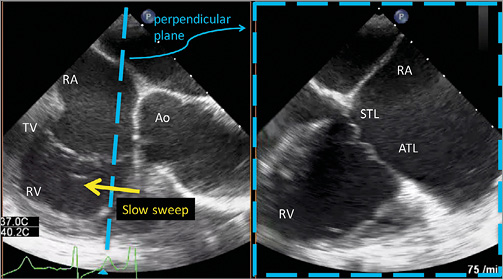
Figure 4. Biplane midoesophageal view of the tricuspid valve by TEE. The left view is orientated along the anteroseptal coaptation line usually at 80-90°. The right perpendicular image is obtained by a slow sweep from the aorta to the valve centre until the large anterior papillary muscle is seen. During this sweep the anteroseptal commissure with its leaflets is displayed. Because the papillary muscle extends chordae to the anterior and posterior leaflets, it is usually a good landmark for the delineation of both leaflets. Ao: aorta; ATL: anterior tricuspid leaflet; RA: right atrium; RV: right ventricle; STL: septal tricuspid leaflet; TV: tricuspid valve
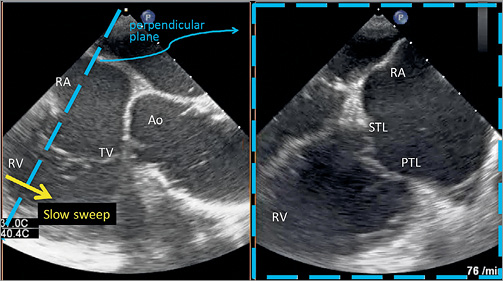
Figure 5. Biplane midoesophageal view of the tricuspid valve by TEE. The left view is orientated along the posteroseptal coaptation line usually at 85-100°. The right perpendicular image is obtained by a slow sweep from caudal to the valve centre until the large anterior papillary muscle is seen. During this sweep the posteroseptal commissure with its leaflets is displayed. Ao: aorta; PTL: posterior tricuspid leaflet; RA: right atrium; RV: right ventricle; STL: septal tricuspid leaflet; TV: tricuspid valve
– commonly orientated along the anteroseptal coaptation line at 60-80° (Figure 4); the septal and anterior tricuspid valve leaflets are displayed if the simultaneous second plane is acquired at the aortic side of the anterior papillary muscle
– commonly orientated along the posteroseptal coaptation line at 70-90° (Figure 5); the septal and posterior tricuspid valve leaflets are displayed if the simultaneous second plane is moved posterior from the anterior papillary muscle (Figure 5)
– a slow sweep along the septal leaflet allows a detailed assessment and measurement of coaptation defects and gaps as well as leaflet length, tethering and restriction, because restricted leaflet movement, especially of the septal leaflet, frequently limits leaflet grasping.
3) 3D-TEE imaging (Figure 6, Moving image 5):
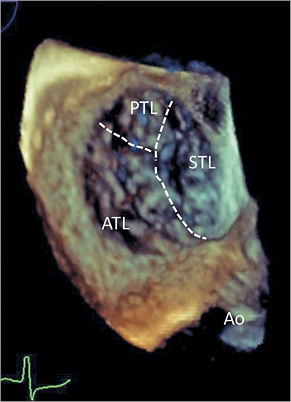
Figure 6. 3D imaging of the tricuspid valve, usually obtained from a mid or lower oesophageal position. Orientation of the image is identical to the transgastric view, facilitating orientation. The aortic valve is rotated to appear at a five o’clock position; the interventicular septum appears on the right side of the image. Ao: aorta; ATL: anterior tricuspid leaflet; PTL: posterior tricuspid leaflet; STL: septal tricuspid leaflet
– the impact of 3D imaging during tricuspid intervention is less clear, as visualisation of the thin leaflets is often insufficient to guide the procedure
– 3D imaging might be helpful for secondary assessment of clip perpendicularity to the coaptation line and for assessing the tissue bridge after grasping and clip closure
– although an en face orientation of the tricuspid valve with the interventricular septum placed inferiorly has been proposed25, we recommend orientating the 3D en face view identically to the transgastric view, which will place the interventricular septum on the right side of the screen with the aorta located at a five o’clock position (Figure 6). This will facilitate periprocedural orientation and image interpretation.
4) Transthoracic echocardiography (Figure 7, Figure 8, Moving image 6, Moving image 7):
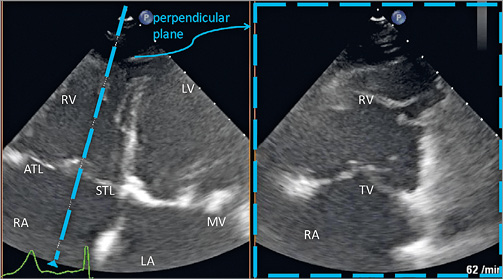
Figure 7. Biplane transapical four-chamber view of the tricuspid valve by TTE. In addition to the assessment of standard RV and TV measurements, this view allows the assessment of leaflet mobility and might be used to support leaflet grasping and to visualise leaflet insertion periprocedurally. The anterior tricuspid leaflet (ATL) is displayed if the transducer is pointing cranially, while the posterior tricuspid leaflet is displayed if the transducer is pointing more caudally; a clear delineation of both leaflets may not always be possible in this view. ATL: anterior tricuspid leaflet; LA: left atrium; LV: left ventricle; MV: mitral valve; RA: right atrium; RV: right ventricle; STL: septal tricuspid leaflet; TV: tricuspid valve
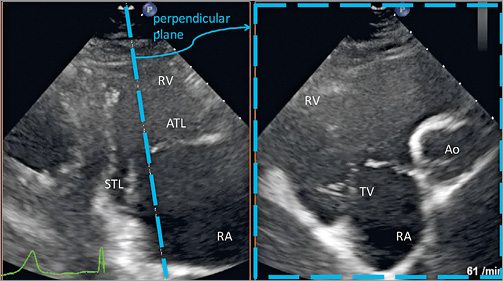
Figure 8. Modified parasternal long-axis view of RV inflow by TTE. This view is often useful to support leaflet grasping as well as visualising leaflet insertion especially in the anteroseptal tricuspid valve commissure. Ao: aorta; ATL: anterior tricuspid leaflet; RA: right atrium; RV: right ventricle; STL: septal tricuspid leaflet; TV: tricuspid valve
– TTE not only provides basic information on right heart size and function, TR severity and estimation of systolic pulmonary artery pressure (sPAP), but frequently also provides important additional views during the procedure
– while it may be challenging to distinguish clearly between anterior and posterior leaflets, these views can be essential to visualise leaflet grasping and leaflet insertion during the procedure.
PATIENT SELECTION
The indication for interventional TR repair should always be determined on an individual basis by an interdisciplinary Heart Team as there are currently no established guidelines for this therapy. During this decision-making process, the following aspects need to be considered:
1) Recommendations of the current pertinent guidelines on the treatment of cardiac valve disease, e.g., issued from European and American societies6,7.
2) Morphology of the tricuspid valve including TR cause and TR severity.
3) Availability of echocardiographic imaging windows of sufficient quality to allow reliable procedural assessment of leaflet grasping and leaflet insertion into the clip device.
4) Right ventricular function and the presence of pulmonary artery hypertension.
5) Presence of trans-tricuspid pacemaker/ICD leads.
6) Concomitant cardiac abnormalities and pathologies relevant to the procedure including presence of prosthetic valves frequently causing deterioration of echocardiographic imaging quality by creating artefacts.
7) The operative risk as assessed by the Heart Team.
Patients with significant TR commonly present with reduced functional capacity (NYHA functional Class II or greater), shortness of breath and/or signs of right-sided heart failure. Patients considered for percutaneous edge-to-edge repair should have persistent symptoms despite optimal medical therapy. A significant number of these patients present with pulmonary artery hypertension, often of post-capillary origin. The TR jet can be used to determine the sPAP by calculating the RV to RA pressure gradient and adding an estimated RA pressure. In patients with massive TR, however, the echocardiographic quantification of pulmonary hypertension will underestimate pulmonary pressures due to the near equalisation of RV and RA pressures. Accordingly, if TR repair is considered, we recommend performing right heart catheterisation during the screening process to assess right heart and pulmonary artery pressures as well as cardiac output to obtain accurate haemodynamic parameters. Although unclear at the present time, it has been suggested that the beneficial effect of tricuspid edge-to-edge repair might be limited to patients without severe pulmonary artery hypertension and without severely elevated pulmonary vascular resistance. In the majority of ongoing studies, an sPAP above 60 to 65 mmHg as well as a significant RV dysfunction are exclusion criteria for interventional treatment of significant TR. These exclusion criteria are based on the surgical experience that the presence of either severe pulmonary hypertension or advanced RV dysfunction is associated with increased operative risk as well as recurrent TR following surgical repair. Therefore, pulmonary hypertension and advanced RV dysfunction are considered markers of poor surgical outcomes. However, it remains unclear whether the surgical experience can be transferred to a percutaneous approach which is performed in beating heart conditions without cardioplegia or circulatory pump support, and without the surgical trauma to the heart and the patient. Furthermore, the risk of acute right heart failure due to a sudden increase in ventricular afterload might be significantly lower with tricuspid edge-to-edge repair than with surgical repair, because complete elimination of the TR is unlikely to be obtained by current interventional TR repair techniques.
Leads of pacemakers or implantable cardioverter-defibrillators (ICD) which cross the TV annulus are often seen in patients with heart failure and significant TR and frequently seem to play a relevant role in the mechanism of regurgitation. Eligibility of patients with trans-tricuspid leads for a tricuspid edge-to-edge repair will be dependent on the origin of the TR, the interaction of the lead with the tricuspid leaflets, and the potential location of clips in relation to the lead(s). A careful echocardiographic screening is mandatory to assess the feasibility of edge-to-edge repair under such conditions. Patients might be considered for tricuspid edge-to-edge repair if the TR is not caused by the pacemaker lead and if mechanical interaction between lead and the clip device is not anticipated. In eligible patients, the regurgitant orifice is usually located at a different valve position from the location where the lead crosses the tricuspid valve annulus. Procedural success and early clinical improvement after edge-to-edge repair might be comparable in these patients to those patients without leads26. However, if the trans-tricuspid pacemaker leads are the primary cause of the TR, e.g., by leaflet restriction, leaflet lifting, leaflet impingement, or scarring of tricuspid leaflets, then tricuspid edge-to-edge repair may not be appropriate for several reasons. These include failure to close the gap caused by the lead, or anticipated mechanical interaction of clip and lead, the consequences of which are currently unknown. Therefore, patients with lead-induced TR currently should not be considered for tricuspid edge-to-edge repair. In such patients lead extraction or replacement with a leadless pacer or a coronary sinus lead might be considered.
Based on the currently available experience, the best candidates for tricuspid edge-to-edge repair may be symptomatic patients at high operative risk with right-sided heart failure symptoms and severe TR. These patients often display a number of comorbidities:
1) previous aortic or mitral valve surgery;
2) previous percutaneous transcatheter aortic valve replacement (TAVR) or mitral valve repair;
3) heart failure with reduced left ventricular ejection fraction (HFrEF);
4) heart failure with preserved left ventricular ejection fraction (HFpEF);
5) atrial fibrillation-induced right atrial enlargement and tricuspid annular dilatation;
6) pulmonary disease with secondary pulmonary hypertension.
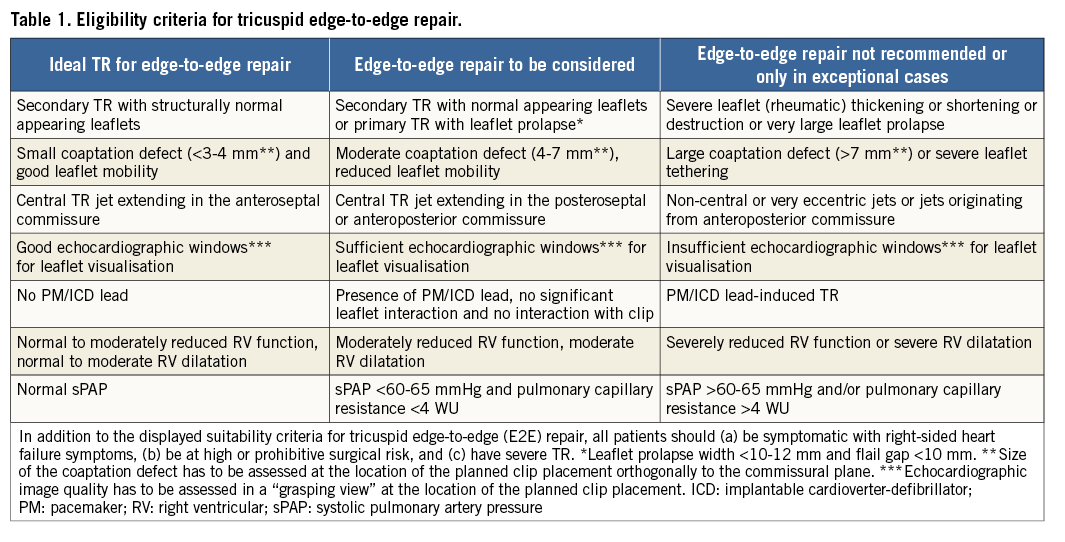
In addition to these patient characteristics, a number of additional criteria will influence the suitability for tricuspid edge-to-edge repair. These criteria, which are predominantly obtained from TEE imaging, are summarised in Table 1. The importance of good echocardiographic visibility for leaflet grasping has been pointed out above; in this regard, it has to be recognised that previous surgical aortic or mitral valve replacement as well as TAVR prosthesis may induce a significant shadowing of the tricuspid leaflets in certain echocardiographic windows. Thus, the suitability for tricuspid edge-to-edge repair may be limited in such patients although they would represent good candidates for an interventional TR repair procedure. Furthermore, given the recent advances in TTE and 3D-TEE probe design and imaging software, the most up-to-date equipment is strongly recommended and considered essential for interventions of the tricuspid valve. Supplementary Figure 1, Supplementary Figure 2, Supplementary Figure 3, as well as the corresponding Moving image 8-Moving image 12, display three patients with challenging anatomic conditions including large coaptation gap and impinging pacemaker leads. Transcatheter edge-to-edge repair which appears to be difficult in such conditions is not recommended with the currently available repair system.
Conclusions
Percutaneous tricuspid edge-to-edge repair appears to be a promising interventional technique for a large number of selected patients with significant TR. Due to the complicated valve anatomy, the complex pathophysiology of TR, and the special haemodynamics of the right heart, a learning process is required to understand these interactions. Nevertheless, interventional edge-to-edge repair on the tricuspid valve carries many similarities with interventional mitral repair using this technique. Procedural and early clinical success will be strongly dependent on appropriate patient selection, appropriate echocardiographic screening for treatment suitability, and adequate echocardiographic visualisation to secure procedural control. The described structured and standardised approach to echocardiographic screening will allow a safe and efficient device placement intraprocedurally and secure procedural success. Furthermore, the described techniques on echocardiographic screening and patient selection will mostly be applicable to other interventional strategies for the treatment of TR.
| Impact on daily practice Percutaneous edge-to-edge repair for symptomatic tricuspid regurgitation is challenging due to the complexity and variability of tricuspid valve anatomy, the difficult echocardiographic visibility of the valve leaflets, and the device steering to the tricuspid valve. The described criteria for patient selection including morphological valve features and the standardised process for echocardiographic screening will support the identification of suitable patients for this therapy. Furthermore, the described strategy for clip placement will help to achieve good procedural results. Thus, the suggestions and recommendations provide a practical guide in the new field of percutaneous tricuspid valve interventions using the edge-to-edge repair technique in daily practice. |
Conflict of interest statement
J. Hausleiter has received speaker honoraria from Abbott Vascular and Edwards Lifesciences. D. Braun has received speaker honoraria from Abbott Vascular. R. Hahn has served as a consultant for Abbott Vascular and GE Healthcare. A. Latib has served on the advisory board for Medtronic, on the Speakers Bureau for Abbott Vascular, on the scientific advisory board for Millipede, and as a consultant for 4Tech, Mitralign, and Millipede. F. Maisano has served as a consultant for Abbott Vascular, Edwards Lifesciences, and Medtronic, and is co-founder of 4Tech. M. Nabauer has received speaker honoraria from Abbott Vascular. P. Lurz reports personal fees from Abbott, during the conduct of the study. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Echocardiographic images of a patient with a large coaptation gap (yellow arrows) of approximately 21 mm in the anteroseptal commissure.
Supplementary Figure 2. TEE images of a patient with a pacemaker lead in the posteroseptal commissure.
Supplementary Figure 3. TEE images of a second patient with a pacemaker lead in the posteroseptal commissure.
Moving image 1. Biplane transgastric view of the tricuspid valve by TEE.
Moving image 2. Simultaneous visualisation of the transgastric short-axis view of the tricuspid valve without (left) and with (right) colour Doppler.
Moving image 3. Biplane midoesophageal view of the tricuspid valve by TEE.
Moving image 4. Biplane midoesophageal view of the tricuspid valve by TEE.
Moving image 5. 3D imaging of the tricuspid valve, usually obtained from a mid or lower oesophageal position.
Moving image 6. Biplane transapical four-chamber view of the tricuspid valve by TTE.
Moving image 7. Modified parasternal long-axis view of RV inflow by TTE.
Moving image 8. A large coaptation gap between the anterior and septal tricuspid leaflets in the biplane midoesophageal view by TEE.
Moving image 9. Interaction of a pacemaker lead with the tricuspid valve, resulting in severe tricuspid regurgitation.
Moving image 10. The same patient (as in Moving image 9) with an interaction of a pacemaker lead with the tricuspid valve, resulting in severe tricuspid regurgitation.
Moving image 11. Interaction of a pacemaker lead with the tricuspid valve in a second patient, resulting in severe tricuspid regurgitation.
Moving image 12. The same patient (as in Moving image 11) with an interaction of a pacemaker lead with the tricuspid valve, resulting in severe tricuspid regurgitation.
A full description of the Moving images can be found in the online version of this paper.
To read the full content of this article, please download the PDF.
Supplementary data
To read the full content of this article, please download the PDF.
Biplane transgastric view of the tricuspid valve by TEE.
The same patient (as in Moving image 9) with an interaction of a pacemaker lead with the tricuspid valve, resulting in severe tricuspid regurgitation. Biplane TEE transgastric views in the short-axis view (left) and the perpendicular two-chamber view (right). Doppler colour flow visualises the severe TR.
Interaction of a pacemaker lead with the tricuspid valve in a second patient, resulting in severe tricuspid regurgitation. Biplane TEE transgastric views in the two-chamber view (left) and the perpendicular short-axis view (right).
The same patient (as in ) with an interaction of a pacemaker lead with the tricuspid valve, resulting in severe tricuspid regurgitation. Biplane TEE transgastric views in the short-axis view (left) and the perpendicular two-chamber view (right). Doppler colour flow visualises the severe TR..
Simultaneous visualisation of the transgastric short-axis view of the tricuspid valve without (left) and with (right) colour Doppler.
Biplane midoesophageal view of the tricuspid valve by TEE. The left view is orientated along the anteroseptal coaptation line usually at 80-90°. The right perpendicular image is obtained by a slow sweep from the aorta to the valve centre until the large anterior papillary muscle is seen.
Biplane midoesophageal view of the tricuspid valve by TEE. The left view is orientated along the posteroseptal coaptation line usually at 85-100°. The right perpendicular image is obtained by a slow sweep from caudal to the valve centre until the large anterior papillary muscle is seen.
3D imaging of the tricuspid valve, usually obtained from a mid or lower oesophageal position. Orientation of the image is identical to the transgastric view, facilitating orientation. The aortic valve is rotated to appear at a five o’clock position; the interventricular septum appears on the right side of the image.
Biplane transapical four-chamber view of the tricuspid valve by TTE. The anterior tricuspid leaflet (ATL) is displayed if the transducer is pointing cranially, while the posterior tricuspid leaflet is displayed if the transducer is pointing more caudally; a clear delineation of both leaflets may not always be possible in this view.
Modified parasternal long-axis view of RV inflow by TTE. This view supports leaflet grasping as well as visualising leaflet insertion.
A large coaptation gap between the anterior and septal tricuspid leaflets in the biplane midoesophageal view by TEE.
Interaction of a pacemaker lead with the tricuspid valve, resulting in severe tricuspid regurgitation. Biplane TEE transgastric views in the short-axis view (left) and the perpendicular twochamber view (right).

