
Growing evidence supports the association between severe tricuspid regurgitation (TR) and poor cardiovascular outcomes. Patients with relevant TR are more frequently hospitalised and have a higher cardiovascular mortality compared to those with no or mild regurgitation1. Surgery is rarely performed for the treatment of isolated severe TR owing to the high in-hospital mortality (close to 10%) reported even in contemporary series2.
The correlation between TR and prognosis appears to go beyond the usually accepted definition of severe TR. There may be a continuous rise of the risk of death with increasing effective regurgitant orifice area (EROA)3. In contrast to left-sided valvular pathologies, patients develop symptoms late and frequently present with large regurgitant volumes. In an attempt to characterise advanced TR better, two additional grades (massive and torrential) have recently been proposed4. Besides incremental prognostic value5, the use of this new grading scheme may also facilitate the appraisal of procedural results.
In this issue of EuroIntervention, Nickenig and co-authors report the two-year results of the TRI-REPAIR prospective multicentre study using this new five-grade scheme assessed by an independent echocardiographic core laboratory6.
Thirty patients (75±7 years of age) with at least moderate TR were treated using the Cardioband™ tricuspid repair system (Edwards Lifesciences, Irvine, CA, USA). Cardioband implantation was successful in all patients and resulted in about a 14% reduction of the tricuspid end-diastolic septolateral diameter that was maintained at 24 months in the 16 patients with available echocardiographic follow-up. This translated into a durable TR reduction, which remained statistically significant in paired comparisons throughout two years (EROA at baseline 0.8±0.5 cm2 versus 0.3±0.2 cm2 at 24 months; p=0.004). A corresponding significant improvement in terms of quality of life and NYHA class was also observed, despite the fact that in this early experience 27% of the patients still had severe TR at two years. The estimated rate of death was 27% (mainly due to non-cardiovascular fatalities), which compares favourably with medical treatment alone (about 60% survival at two years).
As alternative methods, transcatheter leaflet approximations using either the TriClip™ (Abbott Vascular, Santa Clara, CA, USA) or the PASCAL (Edwards Lifesciences) system were also approved early in 2020 in Europe. The 6- and 12-month results of the TRILUMINATE study, which investigated the TriClip device, were overall comparable to the present report (Figure 1),7. Of note, the patients treated with the Cardioband system had more severe TR at baseline, which may explain the slightly higher event rate observed after direct annuloplasty. While not completely absent after clipping, annular reduction seems more pronounced and possibly more durable after direct annuloplasty.
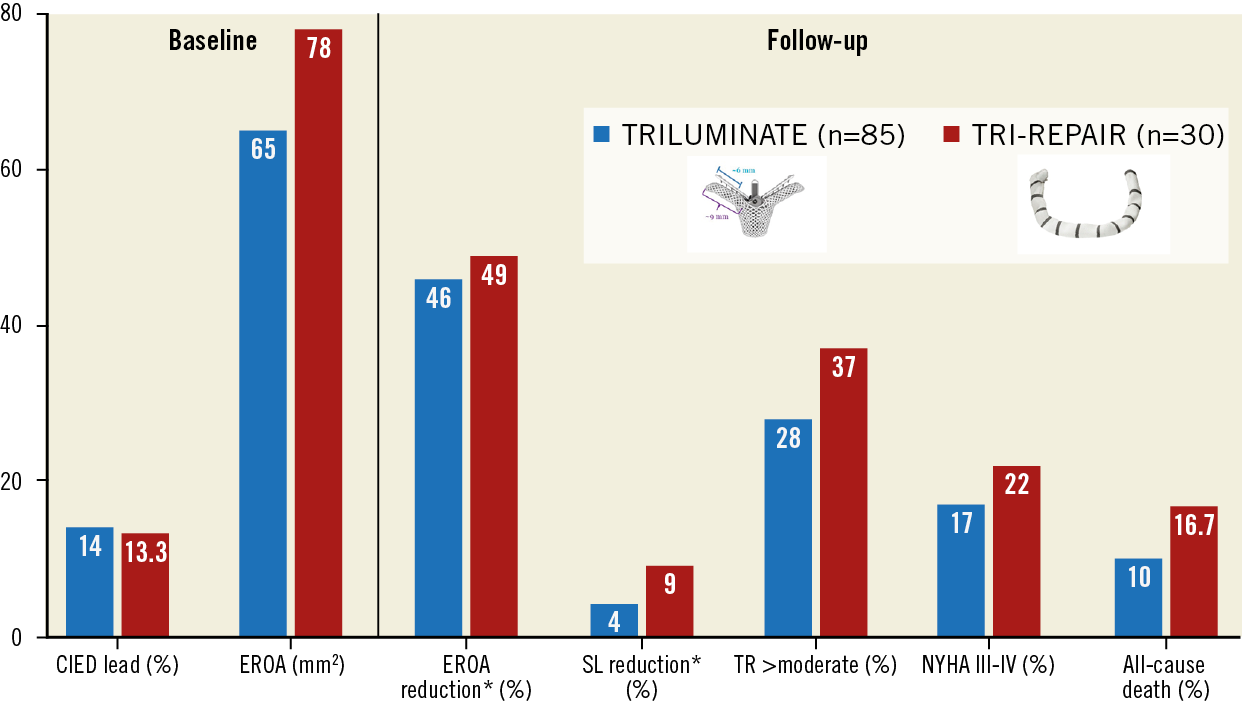
Figure 1. Comparative one-year outcomes of the TRILUMINATE and the TRI-REPAIR studies. * 6-month instead of 1-year outcomes. CIED: cardiac implantable electronic device; EROA: effective regurgitant orifice area; NYHA: New York Heart Association; SL: septolateral; TR: tricuspid regurgitation
Although the Cardioband procedure is more complex and more time-consuming, the following rationale supports its use for transcatheter TR correction in selected patients:
1. TR is of secondary origin in >90% of the cases and relates to annular dilation in the septolateral dimension.
2. Secondary TR severity is directly proportional to the annulus area8.
3. Surgical TR repair techniques without annuloplasty are more prone to TR recurrence9.
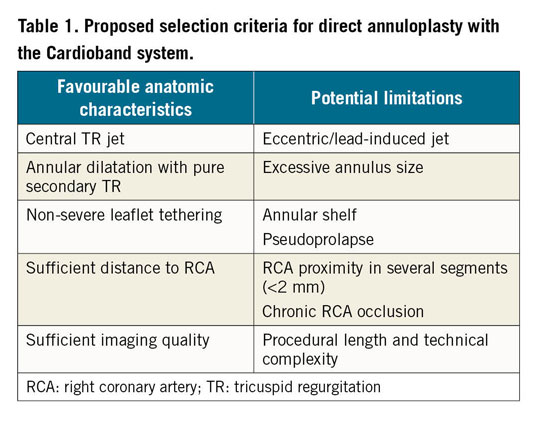
To ensure procedural success, appropriate patient selection using advanced imaging techniques (3D echocardiography and computed tomography) is of paramount importance. Anatomical criteria are proposed in Table 1. Further studies are needed to establish the exact clinical impact, the spectrum of application, as well as the durability of each treatment modality, while synergies between methods may become increasingly important, especially in patients with advanced disease.
Conflict of interest statement
F. Praz reports travel expenses from Edwards Lifesciences, Abbott Vascular, and Polares Medical. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

