Abstract
Background: Severe tricuspid regurgitation (TR) has limited treatment options and is associated with high morbidity and mortality.
Aims: We evaluated the safety and effectiveness of the Cardioband tricuspid valve reconstruction system from the ongoing European single-arm, multicentre, prospective TriBAND post-market clinical follow-up study.
Methods: Eligible patients had chronic symptomatic functional TR despite diuretic therapy and were deemed candidates for transcatheter tricuspid repair by the local Heart Team.
Results: Sixty-one patients had ≥severe functional TR. At baseline, 85% of patients were in NYHA Class III-IV, 94% had ≥severe TR (core laboratory-assessed) with 6.8% EuroSCORE II and 53% LVEF. Device success was 96.7%. At discharge, 59% (p<0.001) of patients achieved ≤moderate TR and 78% had at least one grade TR reduction. At 30 days, all-cause mortality and composite MAE rates were 1.6% and 19.7%, respectively; septolateral annular diameter was reduced by 20%, where 69% of patients achieved ≤moderate TR and 85% of patients had at least one grade TR reduction (all p<0.001). Mid-RVEDD, RA volume, and IVC diameter decreased by 10% (p=0.005), 21% (p<0.001), and 11% (p=0.022), respectively; 74% were in NYHA Class I-II (p<0.001) with improvements in overall KCCQ score by 17 points (p<0.001).
Conclusions: In the TriBAND study, the Cardioband tricuspid system demonstrated favourable outcomes at discharge and 30 days in a challenging patient population with symptomatic ≥severe functional TR. Results showed significant reductions in annular diameter and TR severity, accompanied by early evidence of right heart remodelling and improvements in functional status and quality of life.
Introduction
Tricuspid regurgitation (TR) is a prevalent disease and is dependent on age, sex, and concomitant left-sided heart conditions1,2. The prevalence of ≥moderate functional TR is as high as 23% in heart failure patients2. Greater TR severity is associated with greater cardiovascular morbidity and mortality2,3. A recent epidemiological analysis and preliminary data from interventional studies suggest that TR itself may impact on the clinical sequelae and outcome of patients4. Despite some initial evidence that TR reduction may be associated with favourable outcomes, rigorous endpoint data are still lacking5,6,7,8,9.
Unfortunately, treatment options for TR remain limited. Medical therapy has concentrated on preload reduction, whereas open heart surgery to treat TR is most often performed in patients undergoing left-sided heart surgery10. Isolated TR surgery is infrequently performed given the late presentation and multiple comorbidities, resulting in high perioperative morbidity and mortality rates11,12. Therefore, the focus is on less invasive options to treat TR.
The predominant aetiology of TR is functional, where dilatation of the tricuspid valve annulus occurs in response to either right ventricular or atrial enlargement leading to inadequate leaflet coaptation13. Recently, transcatheter annular reduction was successfully performed with the Cardioband™ tricuspid valve reconstruction system (Edwards Lifesciences, Irvine, CA, USA)5,6,7,14. The single-arm, multicentre, prospective TRI-REPAIR study in patients with moderate or greater TR demonstrated favourable safety and feasibility of the Cardioband tricuspid system which significantly reduced TR, ameliorated heart failure symptoms and was accompanied by low adverse event rates5,6.
To extend the experience of the Cardioband tricuspid system, the TriBAND post-market clinical follow-up study was initiated. This report summarises the 30-day outcomes of the first 61 patients.
Methods
TRIAL DESIGN
The European, single-arm, multicentre, prospective TriBAND post-market clinical follow-up study evaluates the safety and effectiveness of the Cardioband tricuspid system to treat up to 150 patients with functional TR. Herein, we report the outcomes of the first 61 patients enrolled in the ongoing study.
Key inclusion criteria were chronic symptomatic ≥moderate functional TR confirmed by echocardiographic core laboratory and heart failure symptoms with New York Heart Association (NYHA) Functional Class II-IV despite optimal medical therapy including diuretic therapy. Patients were deemed candidates for transcatheter tricuspid repair by the multidisciplinary local Heart Team. Key exclusion criteria were left ventricular ejection fraction (LVEF) <25%; pulmonary arterial systolic pressure >70 mmHg by echocardiography or right heart catheterisation; tricuspid proximal isovelocity surface area (PISA) effective regurgitant orifice area (EROA) ≥2.0 cm2; severe right ventricular dysfunction; tricuspid valve anatomy precluding proper device deployment and function; previous tricuspid valve repair or replacement; presence of trans-tricuspid valve pacemaker or defibrillator leads impinging on the tricuspid valve leaflets as evaluated by echocardiography; and renal insufficiency requiring dialysis or severe kidney renal disease with an estimated glomerular filtration rate (eGFR) ≤25 mL/min/1.73 m2.
STUDY CONDUCT
An independent echocardiographic core laboratory assessed all echocardiograms at baseline, discharge, and 30 days, and an independent clinical events committee (CEC) adjudicated safety endpoints and all-cause mortality. The study was approved by local ethics committees and respective health authorities of the participating countries. All patients provided written informed consent and the study was conducted in conformity with the Declaration of Helsinki, Good Clinical Practice principles, and ISO 14155:2011. The study is registered at ClinicalTrials.gov (NCT03779490).
THE CARDIOBAND TRICUSPID SYSTEM PROCEDURAL PRE-PLANNING
Transoesophageal echocardiography (TEE), cardiac computed tomography (CT), and fluoroscopy were used for screening patient anatomy and procedural pre-planning. All patients qualified by having ≥moderate functional TR by site evaluation. Optimal Cardioband implant size was selected using CT measurement of the tricuspid annular circumference at the phase closest to early diastole5,6,7.
THE CARDIOBAND TRICUSPID SYSTEM PROCEDURE
The Cardioband implant is deployed via transfemoral venous access using the 26 Fr delivery system under TEE and fluoroscopic guidance. Intraprocedural coronary angiography is performed periodically to assess the proximity of the right coronary artery (RCA) to the implant to prevent complications. The first anchor is deployed on the atrial side of the tricuspid valve annulus at the anterior-septal commissure near the aortic root and close to the tricuspid leaflet hinge point. The Cardioband implant is advanced and positioned along the anterior and posterior regions of the tricuspid annulus by deploying a series of anchors in a stepwise manner. Upon full deployment, the implant is optimally contracted using the size adjustment tool to reduce the tricuspid annular diameter and area, thereby reducing TR5,6,7.
TRIAL ENDPOINTS
The primary endpoint is reduction in TR severity between baseline and discharge. Secondary endpoints include TR severity, NYHA Functional Class, EuroQol 5-dimensions 5-level health questionnaire (EQ-5D-5L), and overall Kansas City Cardiomyopathy Questionnaire (KCCQ) at 30 days post implant.
The safety endpoint is a composite of major adverse events (MAEs) defined as cardiovascular mortality, myocardial infarction (MI), stroke, pericardial effusion requiring intervention, coronary artery injury requiring percutaneous or surgical intervention, arrhythmia and conduction disorders requiring permanent pacing, new need for renal replacement therapy, severe bleeding15, non-elective tricuspid valve reintervention (percutaneous or surgical), major access-site and vascular complications, and major cardiac structural complications.
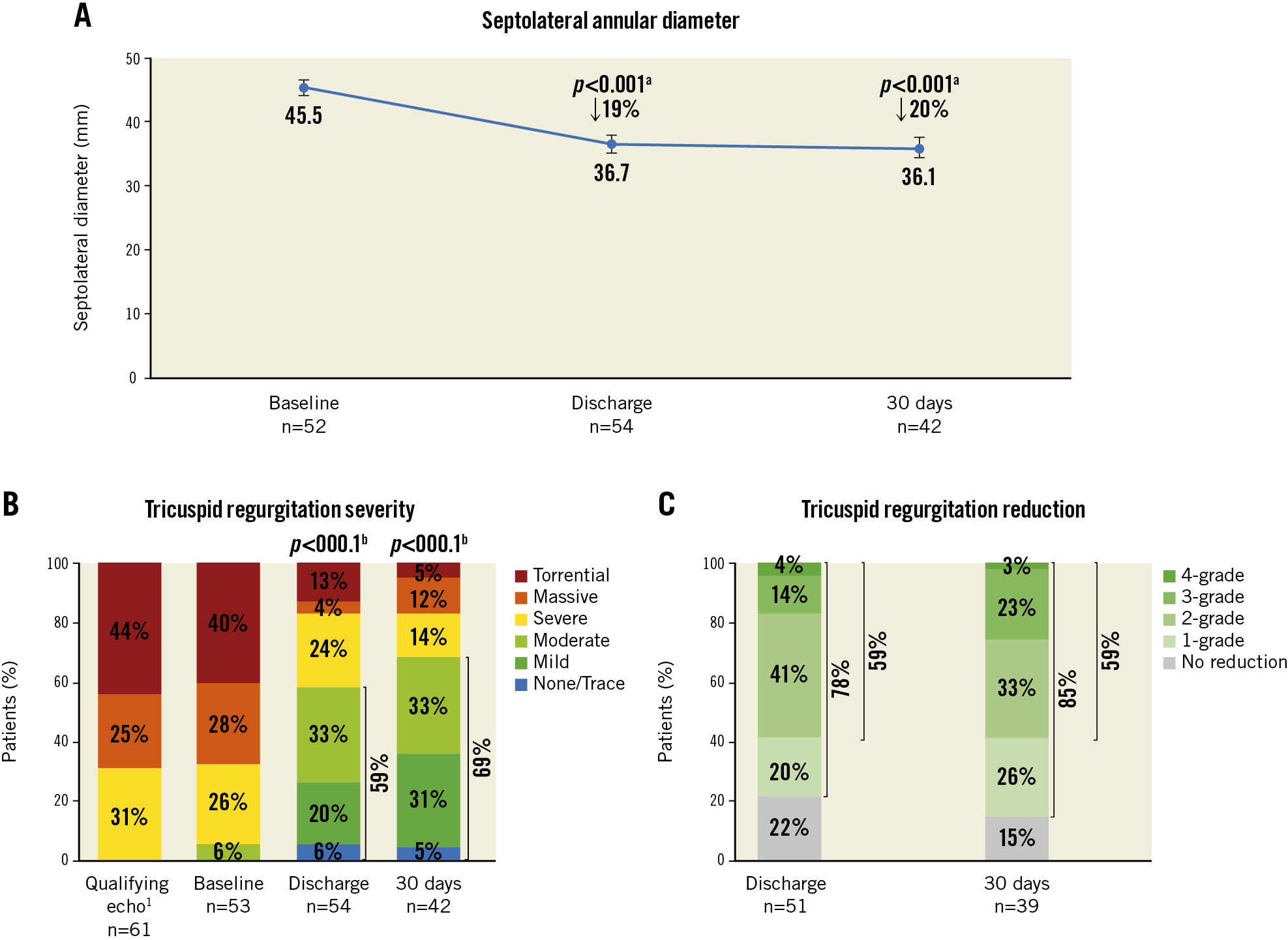
Device success is calculated for patients in whom the device was attempted and is defined as the device deployed and the delivery system successfully retrieved as intended at the time of the patient’s exit from the cardiac catheterisation laboratory (per device). Procedural success was defined as device success with 30% TR reduction in PISA EROA post procedure relative to baseline, and without the need for intervention prior to discharge (per patient). Clinical success is defined as procedural success with no MAEs at 30 days (per patient).
ECHOCARDIOGRAPHY
An independent core laboratory (Cardiovascular Research Foundation, NY, USA) assessed all echocardiograms at baseline, discharge, and 30-day follow-up based on American Society of Echocardiography (ASE) guidelines16. Septolateral annular dimensions were measured from the outer-to-outer borders of the device from an apical four-chamber or RV-focused view in end-diastole. Evaluation of TR was performed using standard two-dimensional colour Doppler and quantitative methods, while TR severity was assessed using the five-grade scheme17,18. Mean vena contracta was calculated as the average of vena contracta widths measured using two orthogonal planes or four-chamber and parasternal RV inflow views. Regurgitant volume and EROA were calculated using PISA and Doppler volumetric methods, whereas LVEF was measured using the biplane method of discs (modified Simpson’s rule)5,6,7.
STATISTICAL ANALYSIS
Analysis was performed on the intention-to-treat (ITT) population. Continuous variables are presented as mean±SD using a paired Student’s t-test, whereas categorical variables are presented as a percentage using the Wilcoxon signed-rank test to compare baseline and post-procedure follow-up periods. Statistical significance was set at p<0.05 as two-tailed tests. Delta, percent change, and p-value were calculated using paired analyses. Paired analyses for all echocardiographic and functional outcomes are provided in Supplementary Figure 1-Supplementary Figure 3 and Supplementary Table 1. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
We report results from the first 61 patients enrolled across 13 European sites between July 2019 and December 2020.
BASELINE CHARACTERISTICS
Mean age was 78.6±5.7 years, 75% were female, and 85% were in NYHA Functional Class III-IV. Mean N-terminal pro-B type natriuretic peptide (NT-pro BNP) levels were elevated to 2,072±1,496 pg/mL. Mean EuroSCORE II (European System for Cardiac Operative Risk Evaluation) was 6.8±10.1% and STS PROM (Society of Thoracic Surgeons predicted risk of mortality) score was 7.1±5.4% (Table 1).
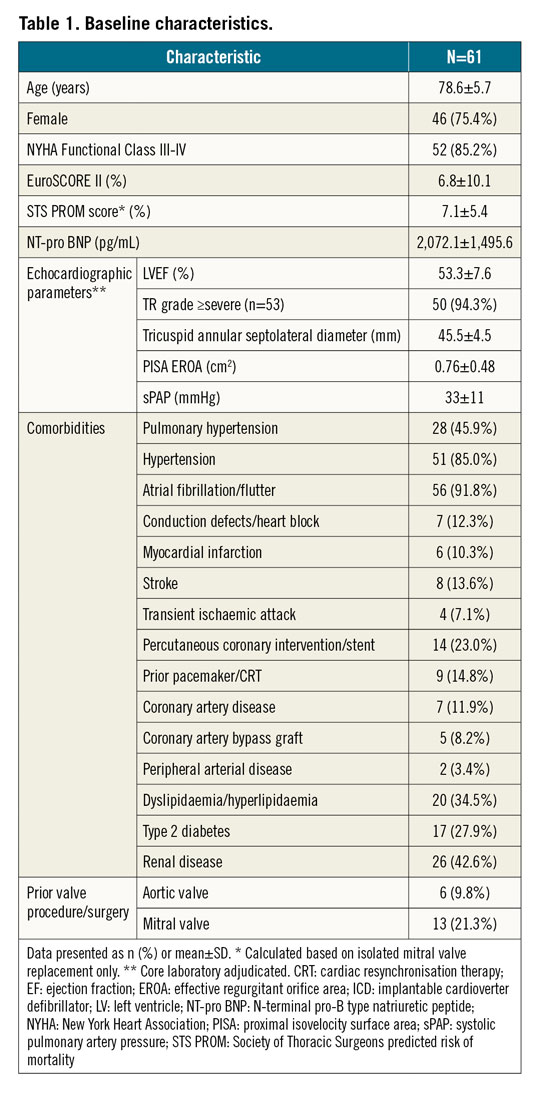
All 61 patients had ≥severe functional TR based on their site-evaluated qualifying echocardiograms; of the 53 patients whose TR was quantified by the echocardiographic core laboratory, 94% had ≥severe TR at baseline (Central illustration). Mean end-diastolic tricuspid septolateral diameter was 45.5±4.5 mm and mean LVEF was 53.3±7.6%. Comorbidities included atrial fibrillation/flutter (92%), pulmonary hypertension (46%), renal disease (43%), type 2 diabetes (28%), prior pacemaker or cardiac resynchronisation therapy (15%), and prior MI (10%) (Table 1).
Central illustration. Septolateral annular diameter reduction and TR severity reduction to 30 days with the Cardioband tricuspid system. A) End-diastolic septolateral diameter at baseline, discharge, and 30 days. Values are mean, 95% confidence interval. B) Qualifying TR severity at screening. Core laboratory adjudicated TR severity at baseline, discharge, and 30-day follow-up. C) Patients with at least one- and two-grade TR reduction at discharge and 30 days, respectively. 1 site-reported; a paired t-test comparing baseline and discharge (n=50) or baseline and 30 days (n=38); b paired Wilcoxon signed-rank test comparing baseline and discharge (n=51) or baseline and 30 days (n=39).
Thirty-day follow-up was performed in 52 of the 61 patients. One patient died before the visit, one patient exited from the study after an aborted procedure, and seven patients had missed or pending visits.
PROCEDURAL AND DISCHARGE RESULTS
The device was attempted in 98.4% (n=60 of 61) ITT patients. In one patient, guidewire perforation of the RCA occurred prior to device insertion and the procedure was aborted. Device success was 96.7% (n=58 of 60). Two patients did not receive the device due to partial deployment of anchors leading to inability to contract the implant (n=1) and poor TEE visibility along with RCA proximity and steep atrial wall (n=1). The procedural success rate was 83.9% (n=26 of 31; EROA unreadable for 30 patients). Procedure time was 202±52 min and fluoroscopy time was 67±27 min. The mean length of hospital stay was 6.5±3.2 days; 88% of patients were discharged home; 12% were discharged to a rehabilitation facility or to a different department in the same hospital. One of these patients died prior to discharge on postoperative day (POD) 18 (Table 2).
ECHOCARDIOGRAPHIC RESULTS
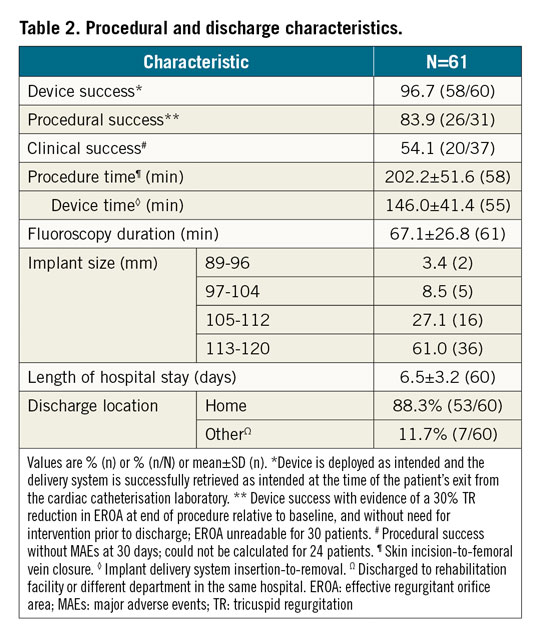
Echocardiographic results were evaluated by the core laboratory for 54 patients at discharge and 42 patients at 30 days (Table 3, Central illustration). The end-diastolic tricuspid septolateral annular diameter was reduced by 19% (p<0.001) at discharge and was stable at 30 days (20%, p<0.001) (Central illustration, A). At discharge and 30 days, 59% (p<0.001) and 69% (p<0.001) of patients, respectively, achieved ≤moderate TR (Central illustration, B). At discharge, 78% of patients achieved at least one grade TR reduction and 59% achieved at least two grade TR reduction. At 30 days, at least one grade TR reduction was achieved in 85% of patients and at least two grade TR reduction in 59% (Central illustration, C).
Early signs of right heart remodelling were evidenced by significant improvements in various echocardiographic indices from baseline to 30 days (Figure 1). Mid-right ventricular end-diastolic diameter decreased by 10% (p=0.005), right atrial volume decreased by 21% (p<0.001), and inferior vena cava diameter decreased by 11% (p=0.022). Although RV TAPSE was significantly reduced at 30 days, there was a significant increase in left ventricular stroke volume by 12% (p=0.007). Similarly, estimated systolic pulmonary artery pressure (sPAP) and cardiac output increased at 30 days (Table 3).
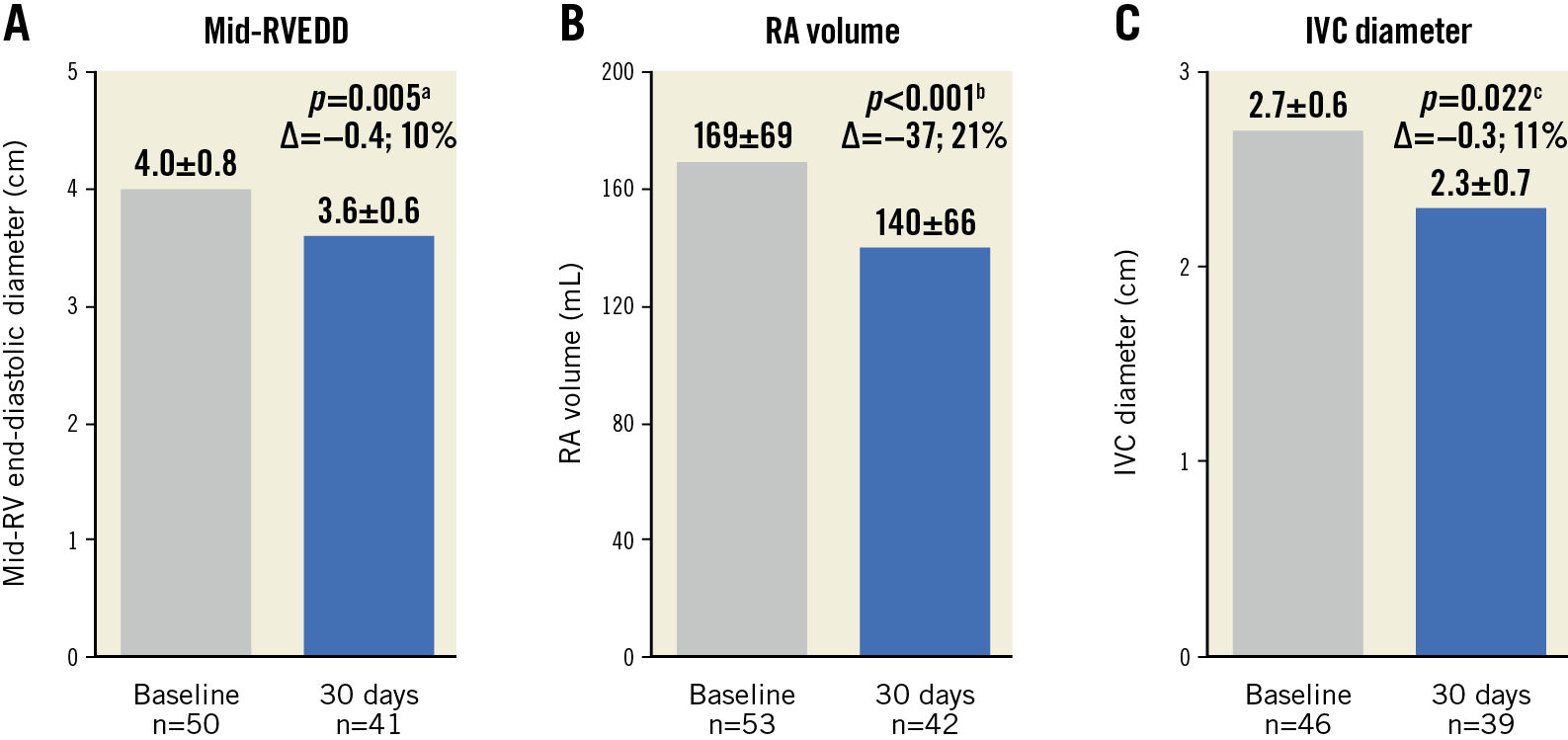
Figure 1. Early evidence of right heart remodelling from baseline to 30 days. A) Mid-right ventricular end-diastolic diameter (RVEDD). B) Right atrial (RA) volume. C) Inferior vena cava (IVC) diameter at baseline and 30 days. Values are mean±SD. Paired t-test comparing a baseline and 30 days (n=37), b baseline and 30 days (n=39) and c baseline and 30 days (n=30).
CLINICAL OUTCOMES
At 30 days, all-cause mortality was 1.6% and the composite MAE rate was 19.7% (Table 4). Clinical success was 54.1% (n=20 of 37; it could not be calculated for 24 patients). One patient developed device- and procedure-related pericardial effusion with severe bleeding requiring drainage. Intraprocedurally, the patient received 100 mL of contrast. The patient also developed uraemic encephalopathy on POD 2, requiring dialysis, which was declined by the patient. The patient was transferred to palliative care and died due to procedure-related renal failure on POD 18.
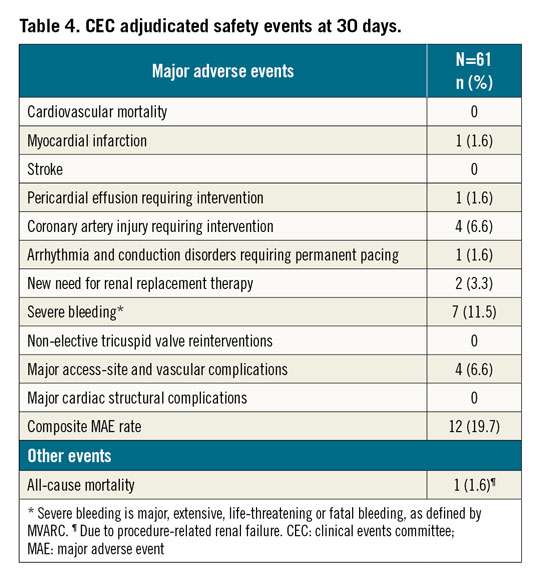
One patient experienced device- and procedure-related MI due to a transient intraprocedural occlusion of the RCA on POD 0 that resolved spontaneously. On POD 5, the patient also experienced procedure-related severe bleeding, and major access-site and vascular complications due to a groin haematoma that also resolved without further treatment.
Four patients experienced coronary artery injuries requiring intervention. One patient experienced a device-related distal RCA occlusion requiring placement of three drug-eluting stents (DES). One patient experienced an RCA flow restriction following contraction of the Cardioband implant that was resolved with one DES and balloon dilatation. One patient developed ST-segment elevations due to an RCA occlusion and underwent percutaneous transluminal coronary angioplasty with successful restoration of blood flow. One patient had extravasation of blood on coronary angiography due to a posterior descending artery perforation from the 13th anchor placement which was repositioned, and successfully treated by placement of a covered stent.
One patient experienced device- and procedure-related intraprocedural bradycardia due to a 3rd degree atrioventricular block, which was treated with a permanent single-chamber pacemaker.
Of the two patients who needed new renal replacement therapy, one patient died (aforementioned) and another patient had procedure-related acute kidney injury requiring dialysis.
Seven patients, all undergoing anticoagulation or antiplatelet therapy due to pre-existing atrial fibrillation/flutter, developed severe bleeding, none of which was fatal and four of which were major access site- and vascular complication-related. One patient developed a pericardial effusion requiring drainage (aforementioned), one patient had a partial perforation of the RCA requiring no intervention, and one patient developed a device-related access-site haemorrhage from the right superficial femoral artery. Three patients had procedure-related right vascular access-site haematomas. One patient had a gastrointestinal bleed not related to the device or procedure.
There were no incidents of stroke, non-elective tricuspid valve reinterventions, or major cardiac structural complications.
FUNCTIONAL AND QUALITY-OF-LIFE OUTCOMES
At baseline, 85% were in NYHA Functional Class III-IV. At 30 days, 74% of patients were in NYHA Functional Class I-II (p<0.001) (Figure 2A), overall KCCQ score improved by 17 points (p<0.001) (Figure 2B), and EQ-5D-5L health score improved by 3 points (p=0.313) (Figure 2C).
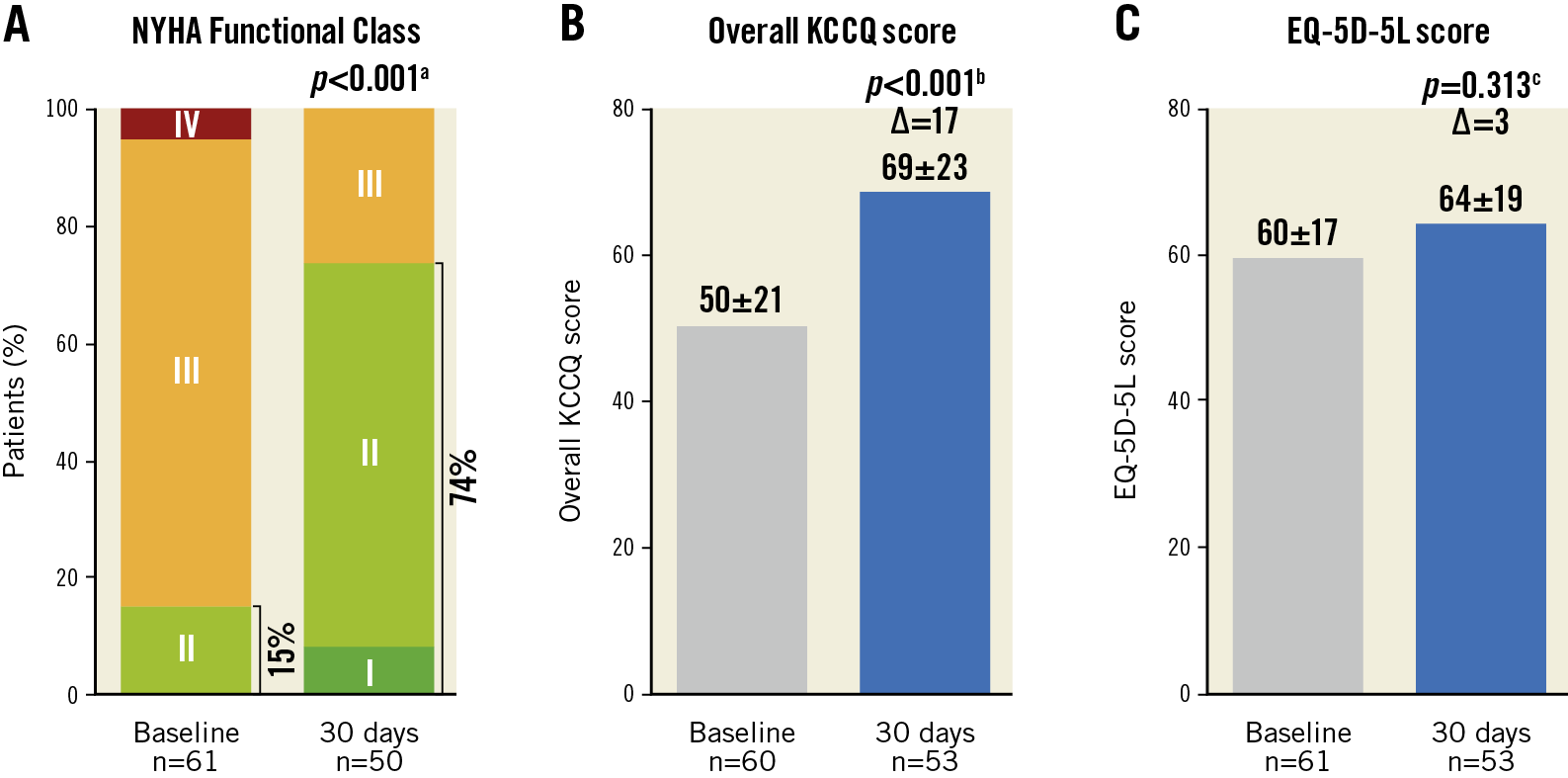
Figure 2. Improvements in functional and clinical measurements from baseline to 30 days. A) NYHA Functional Class. B) Kansas City Cardiomyopathy Questionnaire (KCCQ) score. C) EuroQol 5-dimensions 5-level health questionnaire (EQ-5D-5L) score at baseline and 30 days. Values are mean±SD. Paired t-test or Wilcoxon signed-rank test comparing a baseline and 30 days (n=50), b baseline and 30 days (n=52) and c baseline and 30 days (n=53).
Discussion
These results from the first 61 ITT patients in the ongoing post-market TriBAND study showed reproducibility of the TRI-REPAIR study which led to the CE mark of the Cardioband tricuspid therapy. While the TriBAND study eligibility included moderate or greater TR, 94% of patients enrolled had severe or greater TR at baseline and the results were favourable.
Patients treated in this study had a mean age of 79 years and were severely compromised with multiple comorbidities. Most patients suffered from advanced heart failure, which was driven mostly by right heart disease. Although left ventricular function was preserved, right ventricular function and dimensions were severely affected. Dilatation of the right heart caused pathological septolateral annular dilatation as large as 55.0 mm with secondary malcoaptation of the tricuspid valve leaflets, and subsequent development of functional TR. Core laboratory-adjudicated echocardiographic evaluations revealed that 94% of patients displayed ≥severe TR at baseline (40% torrential TR, 28% massive TR) - an overall challenging cohort of patients.
Device success was achieved in 97% of patients and procedural success achieved in 84%. Contraction of the device led to a 20% reduction of the septolateral annular diameter followed by significant reduction of echocardiographic parameters, such as PISA EROA and mean vena contracta at 30 days. Correspondingly, ≥severe TR was decreased from 94% at baseline to 31% of patients, with 85% having at least one grade TR reduction at 30 days. Similar results were demonstrated in the TRI-REPAIR study at 30 days and were sustained to 2 years5,6. Continued enrolment to 150 patients and follow-up to 5 years in the TriBAND study is ongoing to evaluate long-term outcomes.
Reduction of regurgitant volume was accompanied by relief of right heart overload, as demonstrated by a reduction of right atrial volume and inferior vena cava diameter within 30 days. Similar results were shown in the TRI-REPAIR and TRILUMINATE studies5,6,8. Thus, reduction of regurgitant flow initiates immediate positive reductions in right heart size. The reduction in TAPSE accompanying the reduction in TR is probably a result of both the increase in afterload following reduction in TR, and also the increase in forward flow as evidenced by the increase in sPAP and cardiac output. The right ventricular response to increased afterload is expected, as has been shown in prior studies19. It may be speculated that, although severely diseased, TR-derived right heart disorders are in part functional in nature and can potentially be reversible, providing hope for long-term beneficial effects of the Cardioband tricuspid system.
As a functional consequence of improved right heart performance, left ventricular stroke volume increased significantly. The observed anatomical and functional improvements following the Cardioband tricuspid system implantation led to the clinical benefits in the treated patients with improved NYHA Functional Class I-II from 15% at baseline to 74% at 30 days. Improvement in the overall KCCQ and EQ-5D-5L scores confirm these functional improvements. Similar to the recent studies, in the TriBAND study, even though TR severity was not reduced to ≤mild, heart failure symptoms were significantly alleviated5,6,7,8,9.
There are a few constraints of the Cardioband tricuspid system that are worth mentioning. Firstly, patients with severe leaflet tethering >15.0 mm are ineligible for direct annular reduction therapy20. Secondly, the procedure time continues to improve and warrants further development. In the TriBAND study, the device time was 146 min and total procedure time was 202 min, which is lower compared to the previously reported 255 min in the TRI-REPAIR study, indicative of a shortened learning curve at the participating centres5. Thirdly, Cardioband implantation requires anchor placement between the leaflet hinge points and the RCA, and careful preprocedural planning is necessary. Repeat angiography assists in optimal guidewire placement, while CT scan prevents anchor-RCA perforation and excludes any rare anatomies that are in proximity to the RCA or tricuspid annulus. Even with these measures, in the TriBAND study, RCA injury requiring intervention was observed in 6.6% of patients, comparable to the TRI-REPAIR study5. Fourthly, in this high-risk heart failure population, the bleeding rates were considerable at 30 days and were mostly access site-related, highlighting the need to revisit intraprocedural and site-specific anticoagulation or an antiplatelet regimen. Operator experience with the device and procedure, along with improvements in imaging quality and device technology may further reduce procedure time, while avoiding complications5,6,7. Nonetheless, the promising outcomes of this study are comparable to other transcatheter repair therapies5,6,7,8,9,21.
In summary, the Cardioband tricuspid system uniquely addresses a fundamental pathology of functional TR, i.e., dilatation of the tricuspid annulus. By addressing the annulus and maintaining the native tricuspid leaflets, the Cardioband tricuspid system preserves the option for future interventions such as leaflet repair or valve replacement.
Limitations
This was a non-randomised, non-blinded study. There may be a placebo effect of the intervention, and the results should be interpreted carefully. The sample size is small; more patients and long-term results are needed. TR severity and EROA are missing for many patients as they could not be appropriately evaluated by the echocardiographic core laboratory due to insufficient echocardiograms, warranting additional site training.
Conclusions
The Cardioband tricuspid system demonstrated favourable outcomes at 30 days in the TriBAND study in a challenging patient population with symptomatic ≥severe functional TR. The 30-day results demonstrated significant TR severity reduction through annular reduction, accompanied by early evidence of right heart remodelling and improvements in heart failure symptoms and quality of life. Study enrolment and follow-up are ongoing.
|
Impact on daily practice Tricuspid regurgitation is a prevalent disease and is associated with poor clinical outcomes and limited treatment options. Many of these patients have no alternative therapy options, even with the availability of transcatheter TR intervention. The Cardioband tricuspid system significantly reduced tricuspid regurgitation through annular reduction and consequently reduced heart failure symptoms in a challenging patient population with severe or greater TR. |
Acknowledgements
The authors acknowledge the support of Shekhar H. Deo, MBBS, PhD, Prashanthi Vandrangi, PhD, Suzanne Y. Gilmore, MPIA, Ted E. Feldman, MD, Stella Shao, MS, and Tom Greene, MS.
Funding
Funded by Edwards Lifesciences.
Conflict of interest statement
G. Nickenig, S. Baldus, M. Arnold, U. Schäfer, R.S. von Bardeleben, M. Weber and R.T. Hahn have received speakers’ honoraria or travel/grant support from Edwards Lifesciences. G. Nickenig has received research funding from the Deutsche Forschungsgemeinschaft (DFG), the Federal Ministry of Education and Research (BMBF), the European Union, Abbott, AGA Medical, AstraZeneca, Bayer, Berlin Chemie, BioSensus, Biotronik, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St. Jude Medical, honoraria for lectures or advisory boards from Abbott, AGA Medical, AstraZeneca, Bayer, Berlin, Cardiovalve, Berlin Chemie, BioSensus, Biotronik, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Novartis, Pfizer, Sanofi, and St. Jude Medical, and has participated in clinical trials for Abbott, AGA Medical, AstraZeneca, Bayer, Berlin Chemie, BioSensus, Biotronik, Bristol-Myers Squibb, Boehringer Ingelheim, Cardiovalve, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi and St. Jude Medical. M. Arnold has received lecture fees and honoraria for advisory board activities from Abbott. S. Windecker has received research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, Cardiovalve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson & Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed, and has served as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. He is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration, and is an unpaid member of the Pfizer Research Award selection committee in Switzerland. R.T. Hahn has received speaker honoraria from Abbott Vascular, Edwards Lifesciences and Philips Healthcare, reports consulting for Abbott Structural, Edwards Lifesciences, Medtronic, Navigate, and Philips Healthcare, and is the Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she received no direct industry compensation. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

