Abstract
Aims: We describe the first report of an Edwards SAPIEN valve implanted in a tricuspid bioprosthesis from the femoral vein. We highlight the feasibility of this previously avoided approach and the techniques involved.
Methods and results: A 61-year-old woman with multiple valve replacements for rheumatic heart disease presented with NHYA IV dyspnoea secondary to a severely stenosed tricuspid bioprosthesis. After failed aggressive medical therapy and surgical turn down, an Edwards SAPIEN XT valve was deployed in the tricuspid bioprosthesis via the right femoral vein. Adaptations to the standard transfemoral transcatheter aortic valve implantation (TAVI) technique included: (1) crossing the tricuspid bioprosthesis with a balloon floatation catheter; (2) temporary pacing wire in the coronary sinus rather than the right ventricle; (3) mounting of the SAPIEN XT valve in the reverse orientation to transfemoral TAVI; and (4) fine positioning of the final valve position pre-deployment by 3D transoesophageal echocardiography (3D TOE) alone due to complete radiolucency of the tricuspid bioprosthesis. The procedure was completed without complication and resulted in significant symptomatic improvement.
Conclusions: Deployment of an Edwards SAPIEN valve in a tricuspid bioprosthesis via the femoral vein is feasible and, with careful adaptations to established TAVI techniques, can be performed without complications and with good clinical response.
Introduction
A 61-year-old woman with rheumatic valvular heart disease presented with a gradual deterioration in functional capacity to NHYA class IV, secondary to stenotic degeneration of a tricuspid bioprosthesis (BP) (Moving image 1). She had three previous cardiac operations with mechanical aortic and mitral valve replacements in 1980, a tricuspid porcine BP in 1994 (29 mm Intact Valve; Medtronic Inc, Minneapolis, MN, USA), and repeat mechanical aortic and mitral valve replacements in 2007. Other medical history included atrial fibrillation, severe obstructive pulmonary disease and severe pulmonary hypertension. Clinical examination revealed a diastolic murmur of tricuspid stenosis, non-pulsative hepatomegaly and engorged jugular veins. Echocardiography showed normally functioning aortic and mitral mechanical valves with normal left ventricular function. The right atrium was severely dilated and the right ventricular function was impaired. Systolic right ventricular pressure was estimated at 70 mmHg. The tricuspid BP was severely stenosed (Figure 2A, Figure 2B and Figure 3A) with a peak and mean gradient of 17 mmHg and 9 mmHg, respectively (Figure 3C). The manufacturer indicated the tricuspid BP should have an internal diameter of 25.5 mm, it was measured at 22 mm on transoesophageal echocardiographic (TOE) and 23 mm by computed tomographic scanning. Despite aggressive diuresis, her symptoms continued to deteriorate. After a multidisciplinary discussion, her operative risk was considered prohibitive. It was agreed that an attempt would be made at placing a transcatheter valve in the tricuspid BP via the transfemoral route. Since the tricuspid BP was essentially completely radiolucent, it was decided to perform this procedure under general anaesthesia to permit three-dimensional (3D) TOE guidance.
Bilateral femoral venous access and left femoral artery access was gained. A 5 Fr pigtail catheter was placed in the right atrium for atriography, and a quadrapolar electrophysiology catheter was placed in the coronary sinus to allow rapid pacing (Figure 1A). Attempts to cross the tricuspid BP using a number of guidewires and diagnostic catheters placed within an 8 Fr Mullins™ transseptal sheath (Medtronic Inc., Minneapolis, MN, USA), proved unsuccessful. As we felt that this failure to cross was related as much to the large size of the right atrium as it was to the stenotic tricuspid BP, we attempted to cross the tricuspid bioprosthesis with a 7 Fr balloon wedge pressure catheter (Arrow International, Reading, PA, USA) which was placed within the Mullins™ transseptal sheath to aid with orientation, which proved successful (Figure 1A; Moving image 2). A 0.035 inch Amplatz Extra-Stiff guidewire (Cook Medical Inc, Bloomington, IN, USA) with a tip shaped into a large curve was placed in the right ventricle (Figure 1B). The BP was then dilated under rapid pacing at 140 bpm with a 20 mm NuCLEUS valvuloplasty balloon (NuMED, Hopkington, NY, USA) (Figure 1B, Figure 2C and Figure 2D; Moving image 3). A 26 mm SAPIEN XT Valve was mounted upside-down in comparison with transfemoral aortic valve implantation on the NovaFlex delivery catheter (Edwards Lifesciences, Irvine, CA, USA). Full flexion of the NovaFlex catheter was required to negotiate the acute bend in the right atrium (Figure 1C). Precise positioning of the SAPIEN XT valve into the BP was entirely dependent on 3D TOE (Figure 2E and Figure 2F; Moving image 5 and Moving image 6). The SAPIEN XT Valve was slowly deployed under rapid pacing at 140 bpm with good valve position and no residual leak (Figure 1D, Figure 2G, Figure 2H, Figure 3B; Moving image 7, Moving image 8 and Moving image 9). At one week, the patient is in NHYA class II and has already lost 2 kg of weight (oedema). On transthoracic echocardiography, peak and mean tricuspid valve gradients were 5 mmHg and 3 mmHg respectively (Figure 3D).
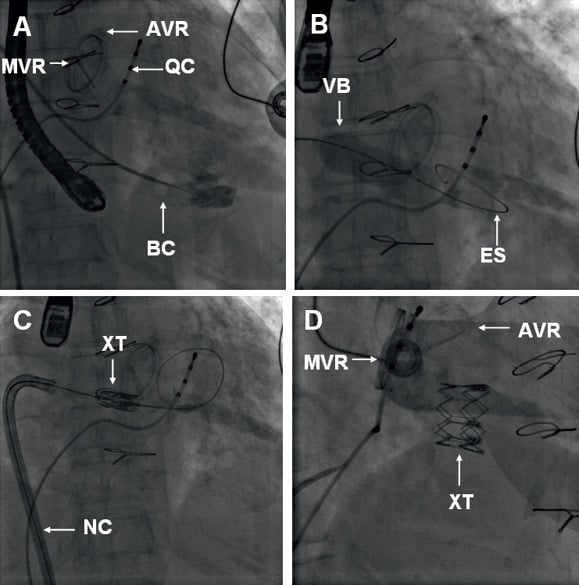
Figure 1. A) Left anterior oblique (LAO) 10 fluoroscopy with the quadrapolar catheter (QC) in the coronary sinus and the positions of the aortic and mitral valve replacements marked (AVR and MVR). Note the absence of radiographic landmarks for the tricuspid bioprosthesis (BP). The tricuspid BP has been crossed with a balloon-tipped catheter (BC) and contrast has been injected through its inner lumen to confirm right ventricular apical position. B) LAO 10 projection of valvuloplasty of the tricuspid BP with a 20 mm balloon (VB) mounted on an extra-stiff guidewire (ES). C) LAO 10 fluoroscopic image at the time of optimal 3D TOE positioning of the SAPIEN XT valve in the tricuspid BP. Precise valve positioning was directed by 3D TOE alone. D) LAO 45 cranial 5 projection showing the final result. AVR: aortic valve replacement; MVR: mitral valve replacement; BP: bioprosthesis; QC: quadrapolar electrophysiology catheter; VB: valvuloplasty balloon; ES: Amplatzer Extra-Stiff guidewire; XT: SAPIEN XT valve; NC: NovaFlex delivery catheter
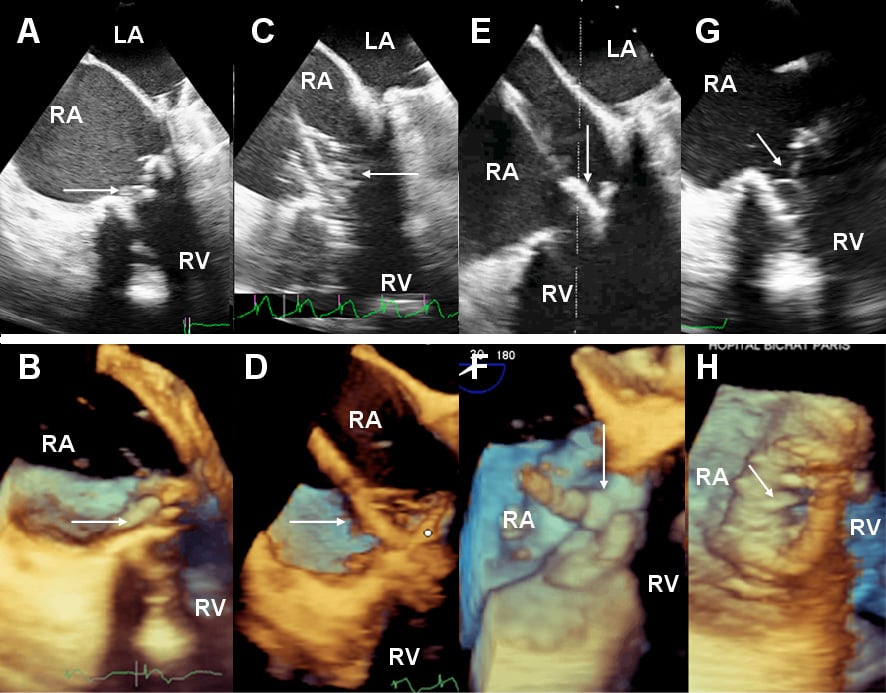
Figure 2. 30° multiplane 2D and 3D TOE views. (A) 2-dimensional (2D) and (B) 3D images of the tricuspid bioprosthesis (BP) before valvuloplasty (arrow). (C) 2D and (D) 3D images of valvuloplasty of the tricuspid BP with a 20 mm balloon inflated (arrow). (E) 2D and (F) 3D images of positioning of the SAPIEN XT valve (arrow) in the tricuspid BP. (G) 2D and (H) 3D images of the deployed SAPIEN XT valve (arrow). LA: left atrium; RA: right atrium; RV: right ventricle
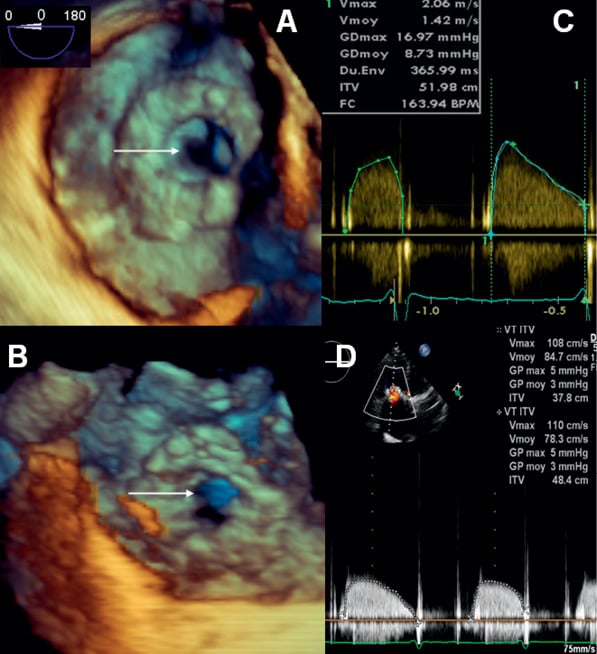
Figure 3. A) En face 3D TOE view of stenotic tricuspid BP before treatment (arrow). B) En face 3D TOE view of SAPIEN XT valve (arrow) within the tricuspid BP. Note the increased orifice size. C and D) TTE tricuspid gradient before (C) and after (D) implantation of SAPIEN XT valve; TTE: transthoracic echocardiography; TOE: transoesophageal echocardiography
Implantation of balloon expandable transcatheter valves in high risk patients with degenerated tricuspid BP has been reported via trans-jugular1,2, and surgical trans-atrial route3. Although the transfemoral route has previously been avoided with the Edwards SAPIEN valve because of the acute angle the delivery catheter must make in the right atrium, this approach has been reported with the Melody valve (Medtronic Inc., Minneapolis, MN, USA) with good outcomes.4 In this series,4 11 out of 15 Melody valves were implanted transfemorally, the remainder via the jugular vein. Reductions in gradients were similar with the Melody valve to those seen in our case, with a mean decrease of the mean gradient from 12.9 mmHg to 3.9 mmHg.
We report the first implantation of an Edwards SAPIEN valve in the tricuspid position via the transfemoral route and demonstrate the feasibility of this approach, taking advantage of the steerable NovaFlex delivery catheter to orientate the device. This case also emphasises the importance of 3D TOE in the positioning of transcatheter heart valves and illustrates how 3D TOE enables valve deployment without fluoroscopy landmarks. Rapid pacing is an important mechanism to ensure accurate placement of balloon expandable valves. In this case, the usual right ventricular pacing could not be achieved with a transvenous pacing lead (as it would be jailed between the percutaneous valve and the stenotic bioprosthesis), or with an epicardial lead (as there was no surgical access planned for the procedure). Hence, pacing was achieved by a quadrapolar pacing catheter placed in the coronary sinus. Progressive inflation of the balloon mounted valve was also integral to correct valve positioning, as previously shown during deployment of transcatheter valves in stentless BP which generally lack fluoroscopic landmarks.5 This case also reminds us of the difficulties of precise internal annulus measurements of BP and the need for multimodality imaging.
Conflict of interest statement
Alec Vahanian is on the Advisory board for Medtronic Inc, Valtech and Abbott Vascular; receives speaker’s fees/honoraria from Edwards Lifesciences, Medtronic Inc and Siemens. Dominique Himbert is a Proctor physician for Edwards Lifesciences and Medtronic Inc. Eric Brochet is a Proctor for Edwards Lifesciences; on the Advisory board for Medtronic Inc and Abbott Vascular. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. 3D TOE view of the degenerated tricuspid bioprosthesis. The valve is viewed from the right atrial aspect and the sewing ring can be clearly seen with the stenotic valve leaflets opening to reveal a reduced orifice area.
Moving image 2. Fluoroscopy of injection of contrast through the balloon tipped catheter to confirm apical right ventricular position.
Moving image 3. Fluoroscopy of valvuloplasty of the tricuspid bioprosthesis with a 20 mm balloon.
Moving image 4. Fluoroscopy of the NovaFlex catheter before centring of the SAPIEN XT valve on the balloon, demonstrating the path the valve must take.
Moving image 5. 3D TOE positioning of the SAPIEN XT valve in the tricuspid bioprosthesis. The tricuspid BP is viewed tangentially (and therefore appears oval) and from the right atrial aspect, with some bubbles appearing in the right atrium from the internal jugular central venous catheter. The proximal part of the SAPIEN XT valve is seen as a bulge on the balloon of the NovaFlex catheter and sits within the orifice of the tricuspid BP. Note that the SAPIEN XT valve oscillates with the motion of cardiac systole.
Moving image 6. Biplane echocardiographic positioning of the SAPIEN XT valve in the tricuspid bioprosthesis.
Moving image 7. Fluoroscopy of the deployment of the SAPIEN XT valve.
Moving image 8. Final result after deployment of the SAPIEN XT valve on fluoroscopy.
Moving image 9. Final result after deployment of the SAPIEN XT valve on 3D TOE. The valves are viewed from the right atrial aspects. The frame of the SAPIEN XT valve can be seen within the sewing ring of the tricuspid BP. The leaflets of the SAPIEN XT valve open to reveal a larger orifice than that seen in Moving image 1.

