
Abstract
Here we describe the first implantation of the novel bicavally anchored Tricento transcatheter heart valve via the transvenous transfemoral access in a 74-year-old woman with severe tricuspid regurgitation and holosystolic hepatic vein backflow. Following successful implantation, caval vein regurgitant volume was reduced leading to symptomatic and clinical improvement at three-month follow-up. The Tricento device represents a promising, novel therapeutic option for patients with severe tricuspid regurgitation who are not candidates for open heart surgery.
Abbreviations
CMR: cardiac magnetic resonance tomography
THV: transcatheter heart valve
Introduction
Tricuspid regurgitation is a very common valvular heart condition which has been directly related to higher morbidity and mortality rates, especially in patients undergoing mitral valve surgery1. However, management of tricuspid regurgitation is still a matter of debate and eventually only a small proportion of patients undergo surgical treatment2.
During recent years, several percutaneous treatment options for tricuspid valve disease have been developed in order to improve quality of life and possibly prognosis in inoperable patients3.
Here, we report the worldwide first implantation of the Tricento® transcatheter heart valve (THV) (NVT AG, Muri, Switzerland) in a multimorbid patient with severe tricuspid regurgitation.
Methods
The Tricento THV is composed of a bicavally anchored covered stent with a lateral bicuspid valve element made of thin porcine pericardial leaflets requiring only a low closing pressure (Figure 1). The stent is made of nitinol and features radiopaque markers allowing exact positioning and orientation during the implantation process. The device aims to abolish the systolic backflow in both the inferior and superior caval veins. The function of the native tricuspid valve remains unaltered. The Tricento THV is delivered through a 24 Fr delivery system via transfemoral access. The implantation is performed top-down and the device is fully repositionable and retrievable up to its final release. Since there is a great amount of inter-patient variability regarding the anatomy of the caval veins and the right atrium, the stent, in its current iteration, needs to be custom made.
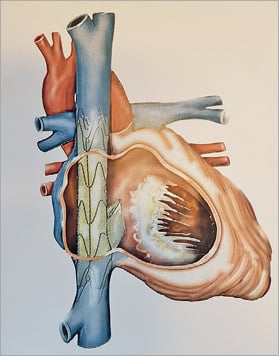
Figure 1. Concept of the Tricento THV. The Tricento is composed of a bicavally anchored covered nitinol stent with a lateral bicuspid valve element made of thin porcine pericardial leaflets.
Results
A 74-year-old woman presented with shortness of breath NYHA Class IV and a history of recurrent hospitalisations for decompensated heart failure. She also suffered from obesity and end-stage kidney disease (glomerular filtration rate of 17 ml/min/kg) with a previous period of dialysis (logistic EuroSCORE 35.2%). Echocardiography revealed preserved biventricular systolic function, severe tricuspid regurgitation with holosystolic backflow in the hepatic veins (Figure 2, Moving image 1) and moderate-severe calcified mitral regurgitation. The patient was discussed in the Heart Team and was deemed to be too high risk for open heart surgery. However, she was considered as a candidate for percutaneous treatment using the Tricento THV. This option was discussed with the patient and her relatives and she agreed to proceed.
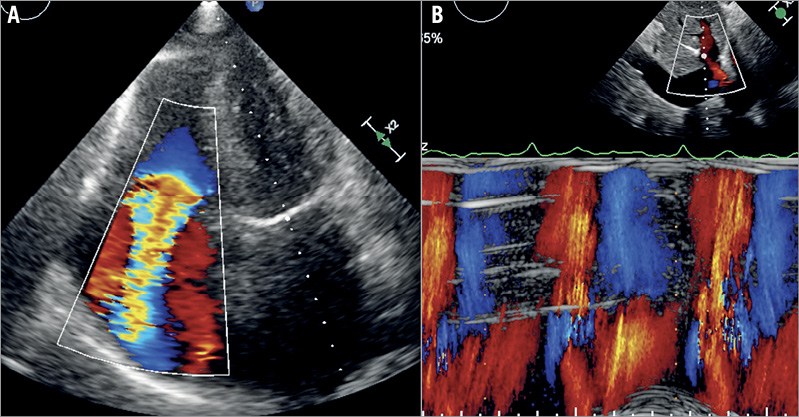
Figure 2. Baseline echocardiography. Transthoracic echocardiography showed severe tricuspid regurgitation (A) with holosystolic backflow in the hepatic veins (B).
Work-up included a cardiac magnetic resonance tomography (CMR) which confirmed severe tricuspid regurgitation with a regurgitant fraction of 64%, holosystolic backflow in the inferior caval vein of 50 ml per stroke, a dilated right ventricle with an end-diastolic volume of 204 ml and preserved right ventricular ejection fraction of 56%. The Tricento prosthesis was subsequently customised based on the CMR measurements of the inferior and superior caval veins, and the right atrium (Figure 3).
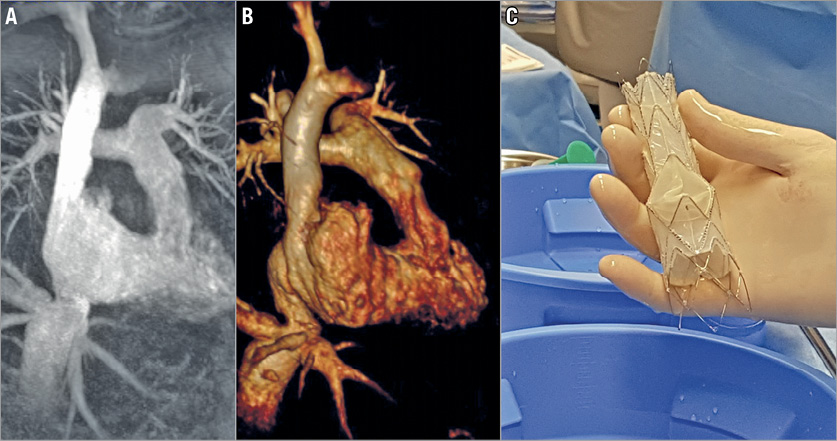
Figure 3. Magnetic resonance images and the custom-made Tricento THV. The Tricento THV (C) was sized based on two-dimensional (A) and three-dimensional (B) magnetic resonance tomography images.
Implantation was performed under general anaesthesia using fluoroscopic and transoesophageal echocardiography guidance (Moving image 2). The device was implanted by gradual, controlled top-down release aiming to position the valve element just above the diaphragm in the right atrium (Figure 4, Moving image 3, Moving image 4). The patient remained completely stable throughout the whole implantation process and there was no need for rapid pacing. Procedure duration was 45 minutes.
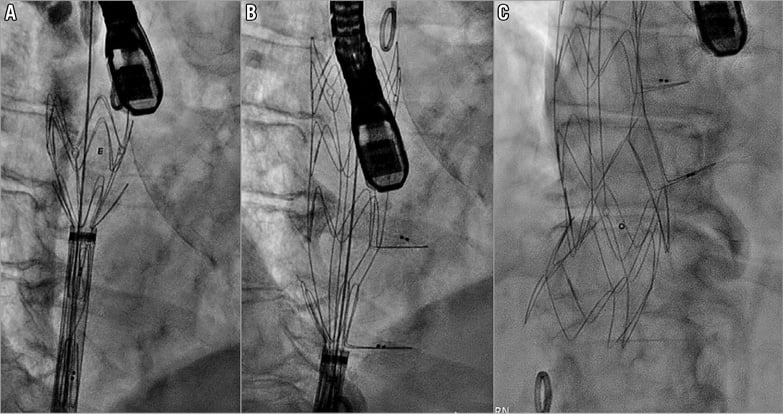
Figure 4. Top-down transfemoral implantation of the Tricento THV. The THV was implanted by gradual top-down release aiming to position the valve element just above the diaphragm in the right atrium. A) Initial deployment of the upper part. B) Deploying the lateral valve element. C) Full implantation of the device.
Directly following the procedure, the patient was extubated and transferred to the regular ward two days later. Besides minor bruising, no access-site bleeding occurred. Her glomerular filtration rate improved from 17 to 32 ml/min/kg. Follow-up CMR indicated that caval vein regurgitant volume was reduced from 50 to 24 ml per stroke, right ventricular function remained unchanged, and cardiac output increased by about 3-5 ml per stroke. The patient’s appetite improved, and her 6-minute walking distance increased from 123 to 158 m, but still remained limited, mainly due to her obesity and reduced general condition. From then on, she did not require any further interventions or hospitalisations for heart failure-related symptoms. She was on a vitamin K antagonist targeting an international normalised ratio (INR) of between 2 and 3. At three months, echocardiography revealed good position and function of the prosthesis with a stable mean transvalvular gradient of 5 mmHg and no transvalvular regurgitation (Moving image 5). There was only a minimal endoleak at the level of the inferior vena cava.
Tragically, four months after the procedure, the patient was admitted to our hospital with multifactorial terminal kidney failure. At that stage, she declined dialysis, and finally passed away secondary to uraemia.
Discussion
Following the success of percutaneous treatment of aortic and mitral valve disease, a number of devices have now been developed aiming to repair the tricuspid annulus, with mixed outcomes. However, the tricuspid valve poses many challenges, such as a complex, variable anatomy, often severely dilated annuli, and associated poor right ventricular function. Therefore, alternative catheter-based concepts such as (bi)caval valve implantation have been utilised and may offer new perspectives in this field4.
The Tricento THV has been specifically developed to treat tricuspid regurgitation. To account for anatomical variability, the stent length and diameter currently need to be customised. In our patient, we aimed to oversize the lower and upper parts of the stent by about 20% in relation to the inferior and superior vena cava, respectively. Currently, the largest stent diameter is 48 mm allowing the treatment of patients with an inferior vena cava diameter of up to 42 mm. Furthermore, the inflow of the hepatic veins has to be considered when planning the procedure. Therefore, a layer of non-covered struts was added to the prosthesis to ensure safe anchoring in the inferior vena cava, even when the patient takes deep breaths in and out. However, a short distance between the diaphragm and the hepatic veins may increase the risk of an endoleak. No implantation in a patient with a previous pacemaker has been performed. Despite being the first-in-man procedure, implantation of the Tricento THV appeared to be safe and straightforward.
Limitations
We are well aware of certain limitations. This is a single-centre case report that does not allow drawing conclusions regarding the overall safety, efficacy, and applicability of this technology. Thus, more prospective data are necessary. In its current version, the Tricento THV needs to be custom made: it may take up to six to eight weeks until the device is ready for implantation.
Conclusions
In patients with severe tricuspid regurgitation who are not considered suitable to undergo open heart surgery, the Tricento THV may reflect a promising and safe therapeutic alternative in order to improve affected patients’ heart failure-related symptoms and quality of life.
| Impact on daily practice A larger case series of implants or a randomised study is required to determine the safety and efficacy of the Tricento THV in patients with severe tricuspid regurgitation who are deemed too high risk for open heart surgery. |
Conflict of interest statement
S. Toggweiler is a consultant and proctor for NVT GmbH. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Baseline transthoracic echocardiography revealing severe (torrential) tricuspid regurgitation due to a large coaptation defect of the leaflets.
Moving image 2. Intraprocedural visualisation of the right atrium and the superior and inferior caval veins.
Moving image 3. Gradual top-down release of the Tricento prosthesis. Opening of the lateral valve element.
Moving image 4. Complete deployment of the Tricento THV.
Moving image 5. Follow-up echocardiography showing both the Tricento THV and the native tricuspid valve.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Baseline transthoracic echocardiography revealing severe (torrential) tricuspid regurgitation due to a large coaptation defect of the leaflets.
Moving image 2. Intraprocedural visualisation of the right atrium and the superior and inferior caval veins.
Moving image 3. Gradual top-down release of the Tricento prosthesis. Opening of the lateral valve element.
Moving image 4. Complete deployment of the Tricento THV.
Moving image 5. Follow-up echocardiography showing both the Tricento THV and the native tricuspid valve.

