Introduction
Transcatheter tricuspid valve replacement (TTVR) is now a promising option for tricuspid regurgitation (TR) patients at high risk for surgery. The LuX-Valve (Jenscare Biotechnology) is a radial force-independent orthotopic TTVR device. The feasibility and efficacy of this device have been reported by several studies123. However, this valve was implanted through right atrial access, where a small incision of the right chest and right atrium is needed. The LuX-Valve Plus valve replacement system is the second-generation version of the LuX-Valve and can be implanted through the jugular vein. The aim of the study was to report the first-in-human results of the LuX-Valve Plus system in patients with severe TR at high risk for surgery.
Methods
This study was performed at the authors’ institutions in China from November 2021 to May 2022. Patients with symptomatic severe TR who were deemed at high surgical risk were included. Patients with severe pulmonary arterial hypertension (systolic pulmonary artery pressure ≥55 mmHg), other valve lesions requiring operative intervention, left ventricular ejection fraction <50%, congenital Ebstein's anomaly or arrhythmogenic right ventricular dysplasia, and untreated severe coronary artery disease were excluded. The study was approved by the local institutional review board and informed consent was obtained from the patients.
The prosthesis of the LuX-Valve Plus system is the same as that of the LuX-Valve1, while the delivery system (33 Fr) is different, permitting a transjugular approach. The available prosthesis sizes were 40, 50 and 55 mm. Procedures were performed under general anaesthesia with transoesophageal echocardiographic and fluoroscopic guidance. A 3-4 cm cut was made in the skin of the right side of the neck and the right jugular vein was exposed. The right internal jugular vein was punctured, a short introducer sheath (36 Fr external diameter and 33 Fr inner diameter) was then placed into the vein, and subsequently the delivery system was advanced. The distal end of the delivery system was adjusted to be in the centre and perpendicular to the tricuspid annulus (Figure 1A). The valve was then released, and the anterior leaflet-grasping clips were expanded (Figure 1B). The delivery system was then withdrawn, allowing capture of the anterior leaflet by the clips. The atrial disc was subsequently deployed, and the valve started to work (Figure 1C). The septal tongue (anchoring component) was then deployed and immobilised by firing a 3-pronged nitinol anchor onto the ventricular septum (Figure 1D). Finally, the valve was completely released (Figure 1E, Figure 1F) and the delivery catheter was drawn back and removed. The processes described above can be seen in Moving image 1. The device could be repositioned and retrieved before the atrial disc was released. Continuous variables were reported as median (25th, 75th percentile), while categorical variables were expressed as frequencies and percentages.
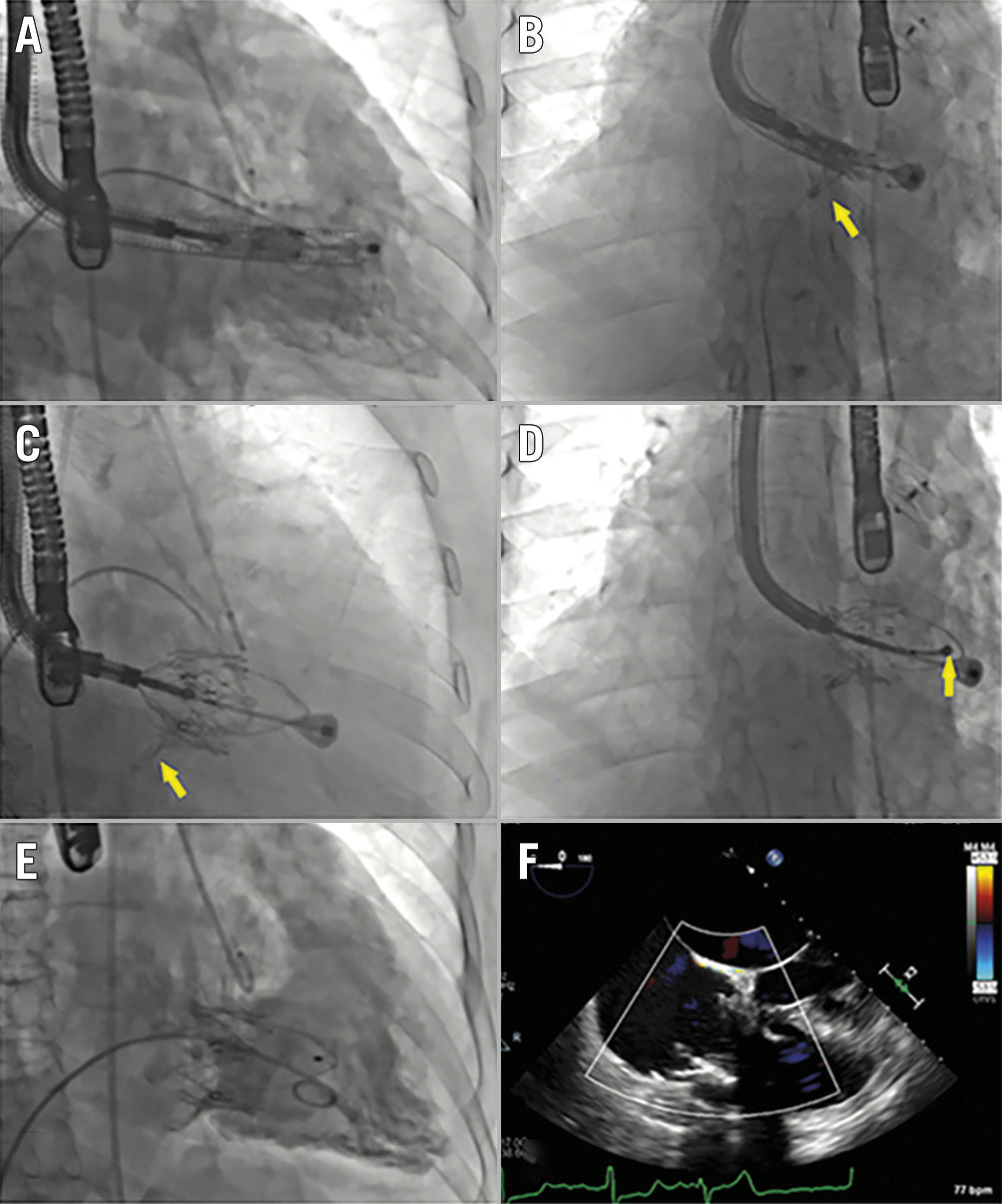
Figure 1. X-ray and echocardiographic images of the LuX-Valve Plus procedure. A) The distal end of the delivery system was adjusted to be right ventricular apex. B) The anterior leaflet-grasping clips (arrow) were released and expanded. C) The anterior leaflet was captured by the clips (arrow). D) The septal tongue (arrow) was then deployed and immobilised. E, F) The valve was finally released.
Results
All patients (mean age 70.0 years, 70% women) were at high surgical risk (mean EuroSCORE [European System for Cardiac Operative Risk Evaluation] II was 11±1%), with New York Heart Association (NYHA) Functional Class III or IV. All patients had more than severe TR at baseline and a mean tricuspid annular diameter of 38.3 mm. Six patients (60%) had undergone left-sided valvular surgery and/or coronary artery bypass graft. The causes of TR were left-sided valvular surgery (50%), permanent pacemaker implantation (10%) and atrial fibrillation (40%).
The device was successfully implanted in all (100%) patients. The peak pressure in the right atrium significantly decreased from 19.0 (11.0, 25.0) to 12.0 (6.0, 21.0) mmHg after implantation (p=0.01). The device time (the duration from the entry of the delivery system to its retrieval) was 37.0 (25.0, 49.0) min. One patient (10%) required pacemaker implantation due to third-degree atrioventricular block 2 days after the procedure. The hospitalisation length was 9 (6-15) days. During the 30-days of follow-up, there was no mortality, no major bleeding, no conversion to surgery or reintervention, no stroke, no myocardial infarction, and no heart failure hospitalisation. The NYHA Class was significantly improved after the procedure as compared with baseline (p<0.05). In 1 patient (10%), who had mild paravalvular leakage at discharge, the TR was reduced to trivial at 30-day follow-up. Thus, all the patients had none/trivial TR at 30 days.
Discussion
The LuX-Valve is a novel TTVR device which has been shown to have good clinical results in several studies123. However, this device is delivered using right atrial access, frequently resulting in postoperative pulmonary complications (PPC)4. The LuX-Valve Plus system is a transjugular TTVR system, implanted through the jugular vein using a 33 Fr delivery sheath. According to our experience in patient screening, the jugular vein of most patients meets the requirements of the procedure (>10 mm). The delivery system has the functions of bending, rotation, and expansion, which allow it to meet the requirements of valve positioning during the procedure and ensure that the valve can be roughly coaxial with the autologous tricuspid valve.
In this first-in-human study, procedural success was achieved in all cases, with no intraprocedural mortality or conversion to open surgery. The discharge echocardiography showed significant TR reduction, and all patients had less than mild TR at 30 days. Additionally, significant improvements in NYHA functional status were observed at 30 days. In particular, no patient developed PPC.
Limitations
This is only a feasibility study of a small sample. The conclusions need to be confirmed by large-sample and multicentre studies. A multicentre premarket clinical trial is being launched.
Conclusions
The LuX-Valve Plus implanted through the jugular vein was feasible in the treatment of TR patients at high risk for surgery and could further reduce trauma and pulmonary complications compared with the first-generation LuX-Valve.
Funding
The study was supported by National Key R & D Program of China (2020YFC2008100) and Ningbo Jenscare Scientific Co., Ltd.
Conflict of interest statement
F. Lu and F. Qiao are consultants for Jenscare Biotechnology. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Animation demonstrating the process of the LuXValve Plus procedure.

