Introduction
Severe tricuspid regurgitation (TR) is frequent and associated with poor outcomes1. Nevertheless, many patients are declined for surgery because of prohibitive surgical risks. In this setting, transcatheter tricuspid valve (TV) intervention may be a less invasive option. Transcatheter edge-to-edge repair (TEER) has been the most used approach so far2; however, transcatheter tricuspid valve replacement (TTVR) might be a more attractive strategy. Indeed, besides providing an alternative for patients whose anatomies are not suitable for TEER (due to large coaptation gaps or short/retracted septal leaflets), TTVR procedures are likely to be less challenging and more reproducible, as demonstrated in the first-in-human trials3.
Methods
The Topaz transfemoral tricuspid heart valve replacement system (TRiCares) is a novel orthotopic self-expanding TTVR system designed with a two-stent frame made of nitinol. The outer stent provides a sealed anchoring into the native tricuspid apparatus while protecting the inner stent, which contains the valve, from any deformation caused by the contraction of the right ventricle (RV). The stiffer inner stent houses the three-leaflet valve made from porcine pericardium, which acts independently of the outer stent, thus allowing it to maintain a circular shape and full valve integrity. The system is delivered via the femoral vein through a 29 Fr steerable introducer, currently with no possibility of recapture. The steerable introducer is first brought into the right atrium and oriented toward the tricuspid annulus. The crimped valve is then advanced to the apex of the RV and deployed in a two-step procedure, first the ventricular part of the valve and then the atrial part. The anchoring mechanism does not rely on radial force but on a layer of anchors located below the annulus level. Therefore, no oversizing of the valve is needed. To date, only one size of the valve is available, which allows treatment of an annulus diameter <45 mm based on diastolic computed tomography (CT) scan measurements.
We herein report the first two cases of compassionate use of the TRiCares Topaz transfemoral tricuspid heart valve replacement system for the treatment of TR. These two cases were approved by the French authorities (Agence nationale de sécurité du médicament et des produits de santé [ANSM]; Ref. No. 2100459) (Figure 1).
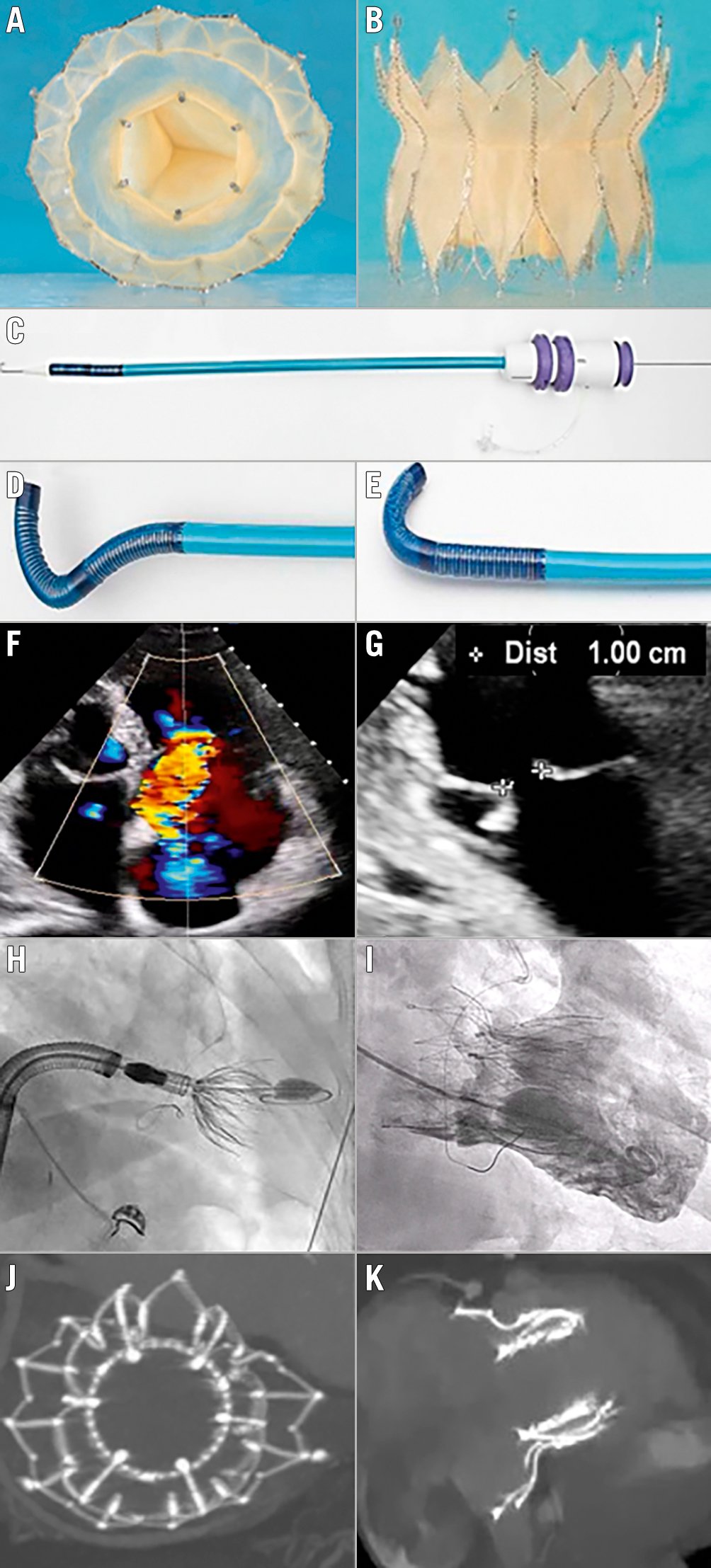
Figure 1. Topaz orthotopic transcatheter tricuspid valve replacement for severe TR. Topaz system: a two-stent design, self-expanding prothesis with 3 porcine pericardium leaflets; the outer stent adapts to the anatomy while the inner stent is more rigid, thus maintaining a circular shape. A) En face view; B) side view. C, D & E) Double-curve steerable sheet for transvenous femoral access. F) Colour Doppler view of the preprocedural torrential tricuspid regurgitation of patient 2 (all the following images are also for patient 2). G) Two-dimensional echographic view of the tricuspid valve coaptation defect. H) Fluoroscopic view of valve deployment. I) Right ventricular angiography immediately after valve deployment. J & K) Post-procedural CT showing the round inner stent linked to the outer stent that conforms to the patient’s anatomy. CT: computed tomography; TR: tricuspid regurgitation
Results
The two procedures were performed in June 2021. The patients were 70-year-old (patient 1) and 86-year-old (patient 2) females. They presented with massive and torrential functional TR1 due to tricuspid annulus dilatation related to chronic atrial fibrillation. However, both were declined for surgery by the local Heart Teams because of prohibitive surgical risks with TRI-SCOREs of 5 (intermediate risk) and 6 (high risk), respectively4, and were not considered suitable for TEER owing to excessive coaptation gaps5.
Interventions were performed under general anaesthesia, using a transvenous femoral approach and guided by fluoroscopy and transoesophageal echocardiography. Procedural success (defined as implantation of the valve at the intended position without migration or significant paravalvular leak) was achieved in both cases, with a short procedure time (18 and 12 minutes, respectively, from delivery system in to delivery system out) and with no adverse events. Immediate haemodynamic results were excellent, with good valve deployment at the intended annular position, a mean transvalvular gradient of 2 mmHg in both patients, no residual TR and no paravalvular regurgitation. The two patients were discharged on day four and, given the setting of atrial fibrillation, remained on a non-vitamin K oral anticoagulant.
Discussion
No death or complication and, notably, no rehospitalisation for heart failure occurred before the post-procedure 3-month follow-up. Transthoracic echocardiography at 3 months showed sustained haemodynamics with no residual TR and an unchanged mean transvalvular gradient (2 mmHg for both patients). However, while there was complete resolution of TR, RV function decreased at three months in both patients (tricuspid annular plane systolic excursion [TAPSE]: 18 mm to 11 mm in patient 1 and 14 mm to 11 mm in patient 2). The three-month CT scan did not reveal any valve thrombosis. Clinical improvement was significant, with an improvement in New York Heart Association (NYHA) functional status from Class III to Class I in both patients. Moreover, in patient 1, the furosemide dose was decreased from 500 mg to none, and the right heart failure symptoms (oedema and jugular congestion) present at baseline were not present at 3 months. Finally, no conduction abnormalities were observed during follow-up.
Limitations
As further investigations are needed, the TRICURE first-in-human trial is planned to start soon.
Conclusions
This first-in-human experience with the Topaz TTVR confirms feasibility and safety, abolishing TR with an effective associated short-term clinical improvement.
Conflict of interest statement
T. Ruf is a consultant and proctor for TRiCares, Abbott Laboratories, and Edwards Lifesciences. P. Blanke is a shareholder of TRiCares. U. Schäfer is a shareholder of TRiCares. H. Treede is a shareholder of TRiCares. R. Gallet is a shareholder of TRiCares. P. Lim is the founder and a shareholder of TRiCares. The other authors have no conflicts of interest to declare.

