
Over the last decade, valve-in-valve (ViV) interventions using transcatheter heart valves (THV) have matured from a proof of concept into an acceptable treatment strategy designed for patients with bioprosthetic valve degeneration. The Global Valve-in-Valve (VIVID) registry represented a milestone in the understanding of the magnitude of the problem and its technical and clinical challenges1. Later, the advances in detailed echocardiography and CT imaging expanded our knowledge and allowed careful preprocedural planning to identify patients at risk for adverse events, such as ostial coronary occlusion. Furthermore, ViV procedures in patients at high risk for re-do open heart surgery were prospectively studied in the aortic ViV nested registry of the PARTNER trial2. This study showed overall low mortality and complication rates, and excellent haemodynamics and quality of life at one-year follow-up. Similar data were demonstrated using the CoreValve®/Evolut™ R system (Medtronic, Minneapolis, MN, USA) in the VIVA prospective registry (data presented at EuroPCR 2017, Paris, France). This compelling accumulated evidence has proved the safety and efficacy of this approach, and the recently released European Society of Cardiology (ESC) guidelines for the treatment of valve disease endorsed a Class 2a recommendation for the catheter-based ViV intervention in the aortic position3.
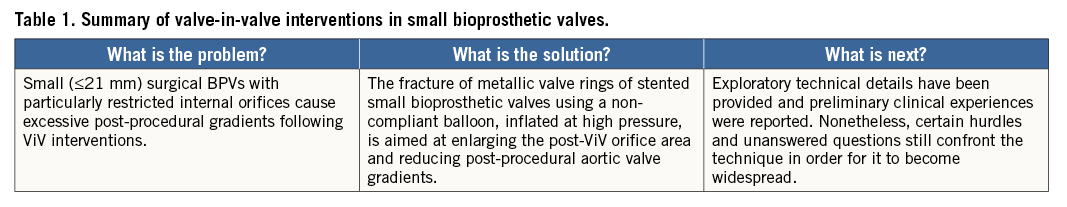
The Achilles’ heel of ViV procedures is small surgical bioprosthetic valves (BPVs). The risks of ostial coronary occlusion and elevated post-procedural transvalvular gradients after ViV are more frequent in patients with small (≤21 mm) BPVs. The frequency of coronary occlusion varies between 2.5% and 3.5% in ViV interventions versus 0.8% in native transcatheter aortic valve implantation (TAVI) procedures, and it is associated with very high (40-60%) short-term mortality1. The preventive management of coronary occlusion begins with meticulous procedural planning. A detailed understanding of the anatomy is based on a comprehensive gated CT analysis to provide the characteristics and dimensions of the BPV and its anatomic environment. Small BPVs (≤21 mm) are also associated with exaggerated post-procedural gradients and augmented risk of patient prosthesis mismatch (PPM). The VIVID registry indicated that TAVI ViV in small (≤21 mm) BPVs was associated with decreased survival. Elevated (≥20 mmHg) post-procedural mean gradients were observed in 26.8% of patients1. All-cause mortality rates following one-year follow-up were: 25.2% vs. 18.2% vs. 6.7%, in patients with a BPV of ≤21 mm, >21 mm to ≤25 mm, and ≥25 mm, respectively (p<0.001). Although the incidence of elevated post-procedural gradients and severe PPM was higher in patients treated with balloon-expandable devices (compared to self-expanding valves), there were no significant differences in rates of all-cause mortality among patients with small (≤21 mm) BPVs treated with either self-expanding or balloon-expandable THV (21.1% vs. 28.7%, p=0.52).
In this issue of EuroIntervention, Adamo et al report 15 cases of ViV intervention with CoreValve and Evolut R THV implanted in small Mitroflow (Sorin [now LivaNova], Milan, Italy) BPVs (19 mm, 21 mm or 23 mm external diameter)4.
The internal orifice measures ~4 mm less than the external diameter. These degenerated valves are extremely challenging due to the risk of coronary occlusion, and the small internal diameter predisposes to elevated gradients and PPM. This small series clearly exhibits the complexity of the problem: coronary occlusion occurred in 26.6% and elevated post-ViV gradients in 26.6% of patients.
The challenge of treating small BPVs led to another emerging solution during ViV interventions. The fracture of the metallic valve rings of stented BPVs using a non-compliant balloon, inflated at high pressure, has been evaluated in vitro and in some preliminary clinical experiences. This topic is currently discussed in two additional papers presented in this issue of EuroIntervention5,6.
In the first paper5, Johansen et al evaluated in bench testing the fracturing mechanics of multiple small (19/21 mm) diameter BPVs, including the Mitroflow, Mosaic® (Medtronic), Trifecta™ (St. Jude Medical, St. Paul, MN, USA) and Magna Ease (Edwards Lifesciences, Irvine, CA, USA) dilated to fracture using a non-compliant balloon. Valves with a polymer frame (Mosaic and Mitroflow) fractured at a lower pressure (8-10 atm) than those with a metal stent (19-26 atm). Trifecta 19 mm did not fracture at all but instead the non-compliant balloon burst at 30 atm and none of the fractured valves showed protruding elements. The same investigators reported their clinical experience by fracturing the BPVs of 10 patients with Mitroflow BPV degeneration6. The mean pressure required for the fracture of the valve in vivo was on average 5 atm higher compared to the in vitro experiments. Balloon fracturing was followed by standard ViV implantation using the S3 or SAPIEN XT valves (Edwards Lifesciences). The mean post-ViV transvalvular gradient was 17±5 mmHg. Minor stroke occurred in one patient (10%) and complete AV block requiring pacemaker in one other case (10%)6.
We think that these preliminary reports are promising but raise critical questions concerning the actual safety and generalisation of the procedure. Although preliminary experiences demonstrate feasibility of the valve fracture manoeuvre, this approach must be further evaluated and standardised. Whether the valve fracture should be performed before or after the ViV is currently unknown. It is as yet unclear whether valve fracture may cause embolisation of friable materials from the BPV ring and/or leaflets. Moreover, whether deliberate ring fracture might increase the rate of annular rupture or coronary occlusion is uncertain. What should be the optimal THV sizing after fracturing is yet to be determined as is the optimal THV to be used during the procedure (balloon-expandable vs. self-expanding THV). Whether valve fracture will increase the need for pacemakers remains unknown, and the long-term results of the procedure should be studied and compared to unbroken BPVs. The consequence of a conceivable bursting of a non-compliant balloon inflated at very high pressure during valve fracture should also be evaluated with caution.
In summary (Table 1), the BPV fracture technique is intriguing but raises fundamental questions. In the future, the design and engineering of stented BPVs might change to adapt for subsequent ViV interventions.
Conflict of interest statement
The authors have no conflicts of interest to declare.

