
Abstract
Aims: Patients with degraded bioprosthetic heart valves (BHV) who are not candidates for valve replacement may benefit from transcatheter valve-in-valve (VIV) therapy. However, in smaller-sized surgical BHV the resultant orifice may become too narrow. To overcome this, the valve frame can be fractured by a high-pressure balloon prior to VIV. However, knowledge on fracture pressures and mechanics are prerequisites. The aim of this study was to identify the fracture pressures needed in BHV, and to describe the fracture mechanics.
Methods and results: Commonly used BHV of small sizes were mounted on a high-pressure balloon situated in a biplane fluoroscopic system with a high-speed camera. The instant of fracture was captured along with the balloon pressure. The valves were inspected for material protrusion and later dissected for fracture zone investigation and description. The valves with a polymer frame fractured at a lower pressure (8-10 atm) than those with a metal stent (19-26 atm). None of the fractured valves had elements protruding.
Conclusions: VIV procedures in small-sized BHV may be performed after prior fracture of the valve frame by high-pressure balloon dilatation. This study provides tentative guidelines for expected balloon sizes and pressures for valve fracturing.
Abbreviations
BF-VIV: balloon fracturing valve-in-valve
BHV: bioprosthetic heart valve(s)
VIV: valve-in-valve
Introduction
Surgical bioprosthetic heart valves (BHV) are used increasingly and more frequently than mechanical valve prostheses1-3. The major disadvantage of BHV is their limited long-term durability due to structural valve deterioration (SVD), resulting in dysfunctional prostheses, which is seen earlier when implanted in younger patients4.
Recently, early failure of the Mitroflow aortic bioprosthesis (Sorin Group [now LivaNova], Milan, Italy) leading to reduced survival has been reported5. The mean time to SVD for the Mitroflow valve was only 3.8 years. The problem with rapid development of BHV dysfunction was seen particularly in the small Mitroflow valve sizes 19 mm and 21 mm.
The standard treatment for deteriorated and dysfunctional biological heart valves is repeat cardiac surgery2,3. However, this carries a significantly raised risk of morbidity and mortality. Insertion of a new heart valve by the transcatheter technique inside the dysfunctional surgical bioprosthesis has therefore evolved as an attractive alternative to redo surgery. This has been named the transcatheter “valve-in-valve” (VIV) treatment6,7. However, insertion of a stent valve into a small surgical BHV with a small orifice will reduce the effective orifice area even further8 and probably have a negative impact on the post-procedural pressure gradient and clinical outcome, including prosthesis-patient mismatch. Thus, transcatheter VIV treatment is not intuitively applicable to small surgical BHV.
This challenge led to a series of in vitro experiments showing that small surgical Mitroflow valves could be fractured by high-pressure balloon dilatation. By this method, the inner valve ring of a small Mitroflow prosthesis made from acetyl could be opened up by a single fracture line, allowing subsequent implantation of a properly sized transcatheter stent valve9. The outer Dacron ring and outside surface were intact with no irregularities or sharp edges after the balloon dilatation. After the in vitro experiments, this VIV therapy with high-pressure balloon predilatation to fracture the surgical valve ring in two patients with small severely dysfunctional aortic Mitroflow bioprostheses was carried out successfully. Furthermore, we have recently performed BHV frame balloon fracturing before VIV (BF-VIV) in a cohort of Mitroflow valve patients, demonstrating BF-VIV to be a promising alternative to repeat cardiac surgery10.
The clinical perspective is obvious. By this novel technique, a high-risk redo heart valve replacement can be converted into a transcatheter VIV procedure with a lower mortality risk, shorter hospital stay, and faster rehabilitation. The concept is promising and can probably be extended and used in other types of small surgical BHV. Extension of this novel treatment concept will require systematic in vitro testing of other surgical BHV in addition to the Mitroflow.
The primary aim of this in vitro study was therefore to test whether commonly used surgical BHV can be fractured by a high-pressure balloon dilatation. Secondly, we aimed to obtain detailed information of the pressure required to induce fracture of the valve ring, the number of fracture zones and the appearance of the fracture zones and other structural components of the biological valves after fracture.
Methods
Atlas® GOLD balloon dilatation catheters (Bard Peripheral Vascular, Inc., Tempe, AZ, USA) with diameters of Ø20 mm, and Ø22 mm were used to fracture a selection of small BHV: Mitroflow 21 mm (Sorin Group); Mosaic® 19 mm and 21 mm (Medtronic, Minneapolis, MN, USA); Trifecta™ 19 mm and 21 mm (St. Jude Medical, St. Paul, MN, USA); and Magna Ease 19 mm and 21 mm (Edwards Lifesciences, Irvine, CA, USA). The valve prosthesis was placed onto the high-pressure balloon, which was gradually inflated until fracture. The inflator and the balloon with the valve were mounted on a custom-made holder, allowing simultaneous visual access to the valve and the balloon pressure gauge (Figure 1A).
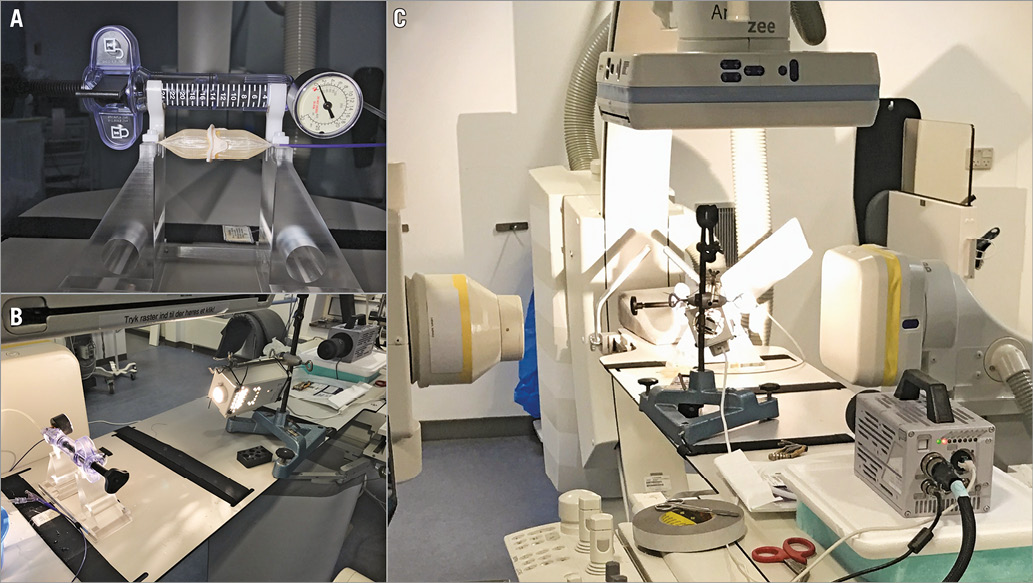
Figure 1. Experimental set-up. A) The inflator, balloon, and valve mounted on the holder. B) & C) Photographs of the experimental set-up. The valve on the balloon dilatation catheter is placed on the fluoroscopic imaging bed along with the high-speed camera. The valve is placed in focus of the biplanar X-ray tubes and detectors.
Furthermore, during inflation, imaging of the balloon-mounted valve and the pressure gauge was acquired at a rate of 200 frames per second with an image resolution of 1,024×1,024 pixels using a high-speed camera (FASTCAM SA3; Photron, San Diego, CA, USA). The entire set-up was positioned within a biplane diagnostic and interventional imaging fluoroscopic system (Artis zee; Siemens, Erlangen, Germany).
The instant of fracture was detected on the high-speed images along with the corresponding balloon pressure. The fluoroscopic images provided the balloon and stent geometry. Moreover, audio recordings (TASCAM DR-40; TEAC, Montebello, CA, USA) were used to assess and characterise the click associated with the fracture. A picture of the entire set-up is seen in Figure 1B and Figure 1C.
DATA ANALYSIS
For each valve type and size the fracture pressure was registered based on the image recording of the pressure gauge. The fracture mechanics were described qualitatively through the fluoroscopy and the high-speed images. Fracture zones were identified from the following examination of the various valve structures exposed through dissection of the valve.
Results
The fracture results are summarised in Table 1 and Figure 2. Table 1 shows that the fracture pressures for the Mosaic valves (19 mm and 21 mm) and the Mitroflow 21 mm valve were in a similar range (8-10 atm). Even though the frame in the Mosaic 19 mm fractured (Figure 2A1), the sutures holding the tissue to the sewing ring were intact. The Mosaic 21 mm demonstrated three fractures at 8, 10, and 12 atm, respectively. This was verified after dissection where the acetal homopolymer stent had three fracture zones (Figure 2B1). A single fracture in the polymer frame was observed in the Mitroflow 21 mm, which was confirmed by inspection after dissection (Figure 2C1). The Trifecta, however, demonstrated much higher resistance to fracture. It was not possible to fracture the Trifecta 19 mm. Instead, the Atlas GOLD balloon burst at 30 atm, which is well beyond the manufacturer’s rated burst pressure of 16 atm. Even though fracture was not possible, the metal frame did experience notable deformation (Figure 2D1). The Trifecta 21 mm did fracture but at a high pressure of 26 atm. A single fracture of the metal stent was observed and confirmed (Figure 2E1, where the fracture is seen at the top of the valve image). The Magna Ease valves (19 mm and 21 mm) both having metal frames were possible to fracture. However, the fracture pressure was 19-21 atm which is about twice the pressure needed to fracture the polymer frames. For the Magna Ease valves, a single fracture was observed in the metal frame (Figure 2F1, Figure 2G1).
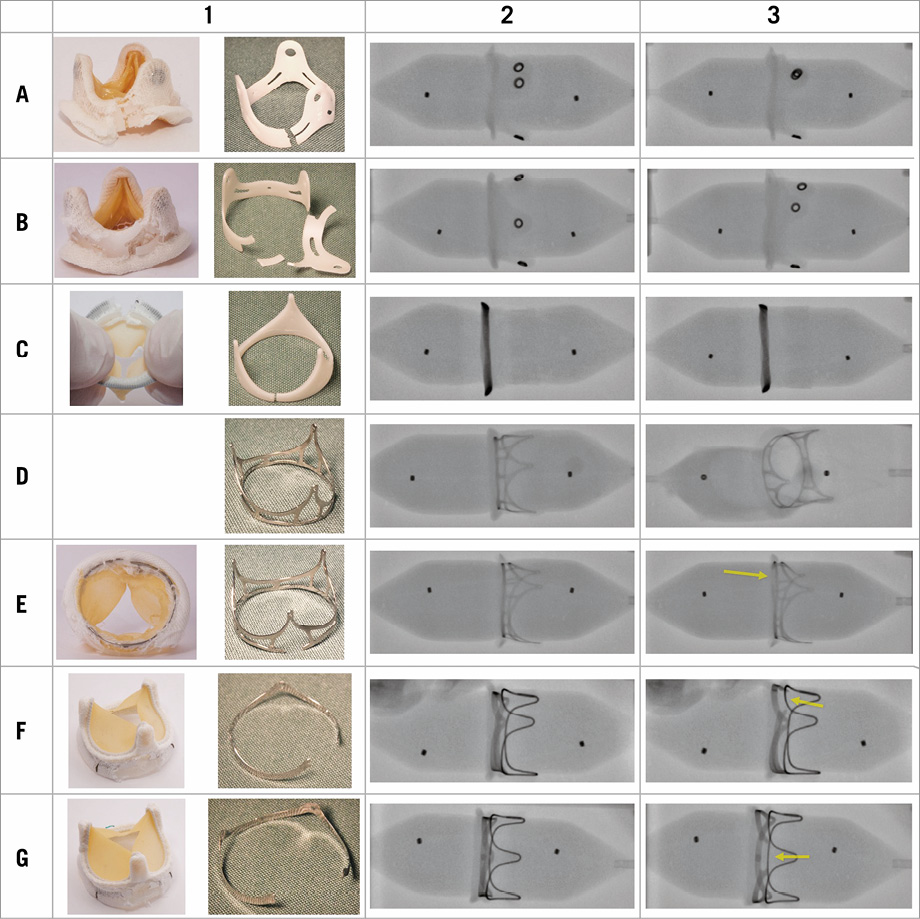
Figure 2. Valve fracture images. Column 1. Images of the valves after the fracture procedure. Left: The fractured valve where the sewing cuff material has been incised to expose the underlying fractured frame structure. Right: The excised valvular frame structure.
Columns 2+3. Fluoroscopy images of the valves on the balloon before (column 2) and after (column 3) the fracture procedure. Row A: Mosaic 19 mm; row B: Mosaic 21 mm; row C: Mitroflow 21 mm; row D: Trifecta 19 mm; row E: Trifecta 21 mm; row F: Magna Ease 19 mm; row G: Magna Ease 21 mm. Notice that the balloon has ruptured in image D3 with the non-fractured Trifecta 19 mm.
None of the fractured valves had sharp elements protruding. For all valves that fractured a notable click could be heard.
Fluoroscopy always demonstrated a clear indentation in the balloon prior to fracture (Figure 2, column 2). Post fracture, the balloon shape even appeared with no or very little indentation (Figure >2, column 3). The fracture zone of the frame structure was difficult to identify on the polymer frame valves, but for the fractured metal frame valves (Trifecta 21 mm and the two Magna Ease valves) it was visible, most notably for the Magna Ease valves (fractures indicated by an arrow in Figure 2).
A fluoroscopic video sequence of the Magna Ease 19 mm valve fracture and the fractures captured by the high-speed camera of the Mitroflow 21 mm and the Mosaic 21 mm along with the attempted fracture of the Trifecta 19 mm valves resulting in balloon rupture can be seen in Moving image 1-Moving image 4.
Discussion
When SVD occurs in patients with BHV, replacement is necessary. For patients who are not eligible for open heart surgery, the VIV procedure is an attractive alternative. However, for small-sized BHV the VIV procedure creates a challenge as it potentially introduces a narrowing. To overcome this, BF-VIV provides a more flexible anchoring site, hence a resulting larger valve effective orifice area can be achieved. However, before engaging in fracturing of implanted degraded BHV in patients, it is compulsory to establish the necessary knowledge on the expected fracture pressures causing the required stress in the frame material to reach the ultimate tensile stress limit, and also to describe how and in what way the valve will fracture. This study provides a detailed and comprehensive investigation on the fracture mechanics of commonly used small-sized BHV. For the investigated valves equipped with a polymer frame structure (Mosaic and Mitroflow), fracture pressure occurred at around 8-10 atm. This is in agreement with a previous study of the Mitroflow valve9 where fracture occurred at a pressure of 16 atm (Mitroflow 19 mm) and 11 atm (Mitroflow 21 mm). Even though clear fracture zones were observed after dissecting the valves, no sharp edges were found to protrude into any of the valve structures. A higher fracture pressure for the smaller-sized valves was expected according to the law of Laplace, which states that the wall tension of a cylindrical structure is the product of the internal pressure and radius. Hence, smaller radii of similar stent geometries (different sizes of the same valve) require a higher balloon pressure in order to establish a wall tension inducing a stress at the ultimate tensile stress limit where fracture would occur, though for the Magna Ease valves this was the opposite. When fracture occurred, a distinct “click” could be heard, and the shape of the high-pressure balloon, as seen on fluoroscopy, changed from an hourglass shape to a more regular cylindrical shape (Figure 2).
The stent geometry of the Mosaic valve differs from the geometry of the Mitroflow in such a way that single parallel structures may fracture at various pressures. This was not observed for the 19 mm Mosaic valve, but for the 21 mm Mosaic valve three fracture sites at three different pressures were observed. This may present a challenge for the fracturing of this valve in patients. Even though indices of fracture have occurred, a total stent ring fracture may not have been achieved. An observable complete fracture feature for the operator could be monitoring the change in shape of the balloon from an hourglass to a cylindrical shape.
For the 19 mm Mosaic valve, the stent experienced a complete fracture at 10 atm balloon pressure. However, even though the stent fractured, the sutures holding the tissue to the Dacron sewing cuff were intact at the fracture site, thus a remaining restriction persisted. However, this residual restraint is not believed to impact on the following TAVI valve deployment and expansion. This should be investigated further.
For the Trifecta valve with a metal frame, fracturing of the 19 mm valve was not possible, though the resultant observed stent deformation indicates plastic deformation, showing that the stress applied exceeded the yield point of the frame material. Fracture of the 21 mm Trifecta, however, occurred at a pressure of 26 atm. This is well beyond the manufacturer’s specified balloon burst pressure rating for the applied balloon. Therefore, VIV therapy with a pre-fracturing of the frame is not recommended for Trifecta valves. The other metal frame valve investigated was the Magna Ease. For this valve, fracture was achieved for both sizes (19 mm and 21 mm) but at about twice the pressure needed for fracturing the valves with polymer-based frames.
To provide a controlled fracture that can potentially be accomplished at a lower pressure than those found in this study (8-10 atm for the polymer stents and 19-26 atm for the metal stents), manufacturers could design the valve frame with a minor indentation. When the accumulated stress introduced by the balloon reaches the fracture threshold, this indentation will serve as the weakest part and fracture will occur here. In this way, through the geometry the manufacturer can control where and at what stress level the fracture will occur and thereby also include design strategies that will minimise or even eliminate the risk of material protruding into the valve structures.
Limitations
One limitation of this study is the number of each investigated valve type. However, it is expected that, due to the low production tolerances for valve manufacturers, similar results would be acquired for repeated measurements. Another limitation is that these detailed investigations are performed in vitro. Once the valves are to be fractured in situ, the surrounding aortic root may act as a reinforcement and affect the pressure needed to accomplish the fracture. In particular, calcified aortic roots may expose a notable reduced compliance, thus introducing a restrictive force acting against the fracturing of the BHV frame structure. These are issues that will and should be addressed in further studies.
Conclusions
Valve-in-valve procedures in small-sized BHV may be performed with a prior valve frame fracture. The results of this study provide tentative guidelines on expected balloon sizes and pressures for valve fracturing of commonly used BHV. Surgical BHV with a frame made of polymer seem suitable for pre-VIV fracture, whereas some metal frame BHV are unsuitable due to extremely high fracture thresholds.
| Impact on daily practice Valve-in-valve therapy for small-sized BHV may be improved by pre-fracturing of the valve frame. Prior in vitro investigation should be carried out to determine the balloon pressure needed for fracturing and to investigate if any material protrusion occurs. Valve fracture can be heard as a “click”, but the operator should verify the fracture by observing a change of shape in the balloon from an hourglass to a cylindrical shape. |
Acknowledgements
The authors would like to thank St. Jude Medical for providing the Trifecta valves, and Medtronic for providing the Mosaic valves for the experiment.
Funding
Snedkermester Sophus Jacobsen og hustru Astrid Jacobsens Fond and Arvid Nilssons Fond are thanked for their generous financial support.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. High-speed imaging of Mitroflow 21 mm fracture.
Moving image 2. High-speed imaging of Mosaic 21 mm fracture.
Moving image 3. High-speed imaging of attempted fracture of Trifecta 19 mm resulting in balloon rupture.
Moving image 4. Fluoroscopy demonstrating fracture of the Magna Ease 19 mm valve.
Supplementary data
To read the full content of this article, please download the PDF.
High-speed imaging of Mitroflow 21 mm fracture.
High-speed imaging of Mosaic 21 mm fracture.
High-speed imaging of attempted fracture of Trifecta 19 mm resulting in balloon rupture.
Fluoroscopy demonstrating fracture of the Magna Ease 19 mm valve.

