
Introduction
Transcatheter aortic valve-in-valve (ViV) implantation has evolved as a valuable alternative to surgical redo operations for degenerated surgical bioprostheses1. The variety of surgical and transcatheter prostheses as well as patient-related and procedural factors in clinical routine make randomised trials in this context virtually impossible. To compensate for the lack of these data, in vitro measurements of ViV hydrodynamics have been increasingly implemented and have helped to understand the influence of prosthesis type, size and implantation height on the procedural result in ViV implantation2,3. In this study, we aimed to assess in vitro hydrodynamic performance of the novel NVT Allegra™ (NVT GmbH, Hechingen, Germany) in different surgical aortic valve bioprostheses. Additionally, we analysed the haemodynamic outcomes of the first four patients undergoing TAVI with this transcatheter heart valve (THV) for the treatment of degenerated surgical aortic valve bioprostheses.
Methods
IN VITRO VALVE-IN-VALVE ANALYSIS
In vitro hydrodynamic performance was investigated using the self-expanding 23 mm NVT Allegra THV in five surgical heart valves (SHVs) with nominal sizes of 23 mm (Figure 1). The NVT Allegra is a self-expanding THV prosthesis, which has recently received CE-mark approval.
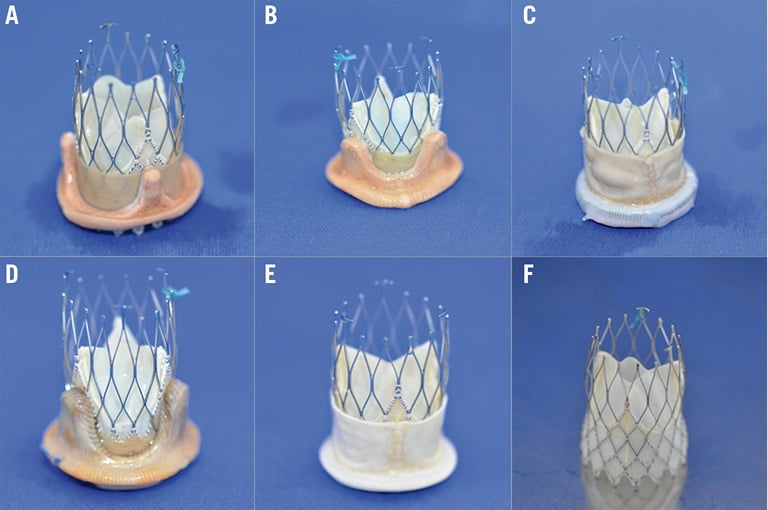
Figure 1. Valve-in-valve pairings using the NVT Allegra with five different valves. A) PERIMOUNT. B) Mosaic. C) Mitroflow. D) aspire. E) Trifecta. F) NVT Allegra.
For in vitro assessment, all SHVs were positioned in Plexiglas aortic root compartments with three valvular sinuses. THV valve size and implantation height were based on recommendations provided by the manufacturer. The resulting ViV configurations were warmed up to a physiological temperature of 37°C to ensure proper stent expansion. Hydrodynamic measurements in this study were performed with saline solution using a pulse duplicator developed at the Department of Cardiovascular Engineering at the Helmholtz Institute, Aachen, Germany4. The following parameters were obtained: a) mean systolic pressure difference (mmHg), b) effective orifice area (cm²), c) leakage volume (% of stroke volume), and d) total regurgitation (% of stroke volume). Measurements were performed at a physiological cardiac output of 5 l/min, repeated twice, and data were collected as a mean with standard deviation across five cycles.
FIRST CLINICAL EXPERIENCE
Four patients with degenerated bioprostheses were treated via a transfemoral approach with the 23 mm NVT Allegra as previously described5. All patients underwent preprocedural evaluation. Afterwards, all patients were discussed by the local multidisciplinary Heart Team at the University Heart Center in Hamburg, Germany.
Results
IN VITRO HYDRODYNAMICS
Mean systolic pressure gradients, effective orifice areas and regurgitation after ViV implantation with the 23 mm NVT Allegra are shown in Figure 2. Mean pressure gradients ranged between 5.0±0.1 and 8.4±0.1 mmHg; the lowest gradients were found in the Trifecta™ (St. Jude Medical, St. Paul, MN, USA). All ViV pairings resulted in effective orifice areas above 1.5 cm2. Both leakage volume and total regurgitation were found to be highest in the ViV configuration with the aspire™ prosthesis (Vascutek Ltd, Inchinnan, UK). The lowest degree of both leakage volume and total regurgitation was observed for the ViV pairing using the Trifecta with 2.3±1.1% and 5.1±1.3%, respectively (Figure 3).
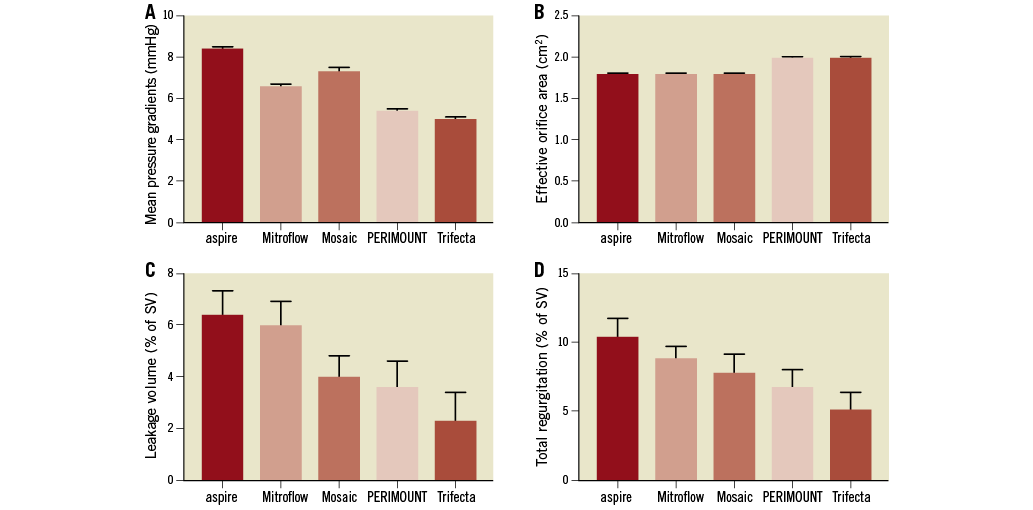
Figure 2. Hydrodynamic results. Mean systolic pressure gradients (A), effective orifice areas (B), leakage volumes (C) and total regurgitation (D) of the 23 mm NVT Allegra in the different surgical bioprostheses.
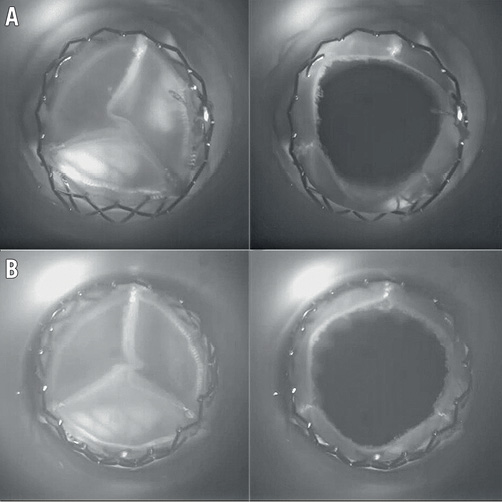
Figure 3. High-speed camera images obtained during in vitro ViV testing during end systole and diastole. A) Mosaic. B) Trifecta. Minimal “pin-wheeling” and no leaflet/stent-frame contact are visible.
FIRST IN HUMAN APPLICATION
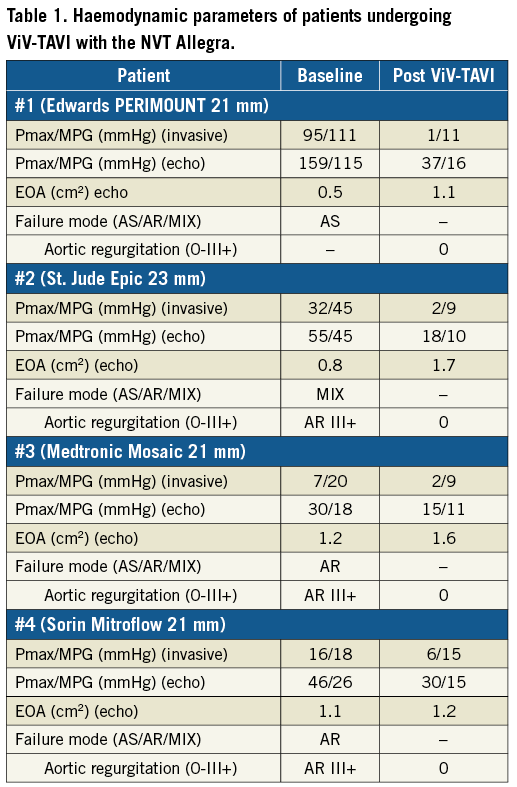
In a small subset of high-risk surgical patients (n=4, log. EuroSCORE 35.8±15.5%; STS-PROM 7.4±6.1%, age 81.2±4.7 years, all female) with degenerated aortic bioprosthestic valves (PERIMOUNT 21 mm [Edwards Lifesciences, Irvine, CA, USA], Epic™ 23 mm [St. Jude Medical], Mosaic® 21 mm [Medtronic, Minneapolis, MN, USA] and Mitroflow 21 mm [Sorin, Milan, Italy]), NVT Allegra prostheses were implanted transfemorally (Table 1). All procedures were performed under local anaesthesia; mean procedural duration was 52±7 min. ViV-TAVI using the Allegra led to improved haemodynamic results. Post-procedural mean (invasive and) echocardiographic analysis showed mean transvalvular gradients below 20 mmHg. During ViV-TAVI, neither coronary obstruction nor significant (paravalvular) leakage occurred. All patients were discharged home without any complication after a median of seven days.
Discussion
In this study, ViV function of a novel self-expanding THV with supra-annular leaflet position, namely the NVT Allegra, was evaluated in five different surgical prostheses with a label size of 23 mm. Comparable with other self-expanding THVs, the NVT Allegra showed good hydrodynamic properties with regard to mean systolic pressure gradients as well as effective orifice areas and regurgitation at a neutral position.
In addition, in a small series of ViV-TAVI (n=4), implantation of the 23 mm NVT Allegra in degenerated bioprostheses with different modes of failure resulted in favourable haemodynamics with low mean transprosthetic gradients, as determined by invasive haemodynamic assessment and echocardiography, and no aortic regurgitation. VARC-2 criteria for device success were met with mean aortic valve gradients <20 mmHg in all cases. An ancillary hydrodynamic in vitro analysis of the 23 mm NVT Allegra within a 21 mm Sorin Mitroflow SHV was performed to complement the clinical data: this showed that mean systolic pressure gradient (12.2±0.2 mmHg) as well as effective orifice area (EOA 1.4 cm2) in vitro were well in line with the results in patient #4.
Limitations
Several limitations in this study need to be acknowledged: the surgical bioprostheses used in the in vitro analysis were unimplanted and not degenerated, therefore lacking calcification and/or pannus, both of which may cause incomplete THV expansion and thus paravalvular leakage in ViV-TAVI. Second, the effects of THV position on hydrodynamic outcome or stability were not evaluated in this study. In this analysis, all THVs were implanted at the optimal position; however, in clinical routine this may not always be achievable. Finally, surgical heart valves in the in vitro analysis were not identical to those in the clinical ViV cohort, limiting the direct comparison of both.
Conclusion
Both of the observed hydrodynamic properties of the NVT Allegra in our early clinical experience are encouraging and indicate a potential role for this novel THV in valve-in-valve TAVI. Given the small size of this analysis, it remains for prospective studies such as the recently initiated VIVALL trial (NCT03287856) to evaluate the clinical performance of the NVT Allegra for the valve-in-valve indication.
| Impact on daily practice With the lack of randomised studies, in vitro valve-in-valve analyses have gained increasing importance, providing reliable information on optimal THV choice and THV position. The NVT Allegra, a novel supra-annalur THV, displayed good performance with regard to mean pressure gradients and regurgitation in different surgical prostheses in benchmark tests. These results were confirmed in the clinical setting. The results of this analysis strengthen the role of in vitro valve-in-valve tests as a precursor to clinical use of novel THVs and indicate the safety and feasibility of ViV-TAVI with the NVT Allegra. |
Acknowledgements
In vitro measurements were performed with the support of the company ac.biomed GmbH (Aachen, Germany).
Conflict of interest statement
A. Sedaghat has received travel grants from NVT. J.M. Sinning and N. Werner have received speaker honoraria as well as research support from Medtronic. U. Schäfer and S. Toggweiler are consultants for NVT. G. Nickenig has received speaker honoraria from Edwards Lifesciences as well as Medtronic. L. Conradi is a consultant for Edwards Lifesciences and has received speaker honoraria from Medtronic.

