Introduction
The NVT Allegra transcatheter heart valve (NVT) (New Valve Technology, Hechingen, Germany) received CE-mark approval in March 2017. The valve is composed of a self-expanding, nitinol stent frame with a sewn-in supra-annular bovine pericardial heart valve1. Currently, there are three sizes (23 mm, 27 mm, and 31 mm) available, all delivered through an 18 Fr sheath.
Methods
Prospectively collected data between April 2017 and January 2018 from five European centres in Spain and Switzerland were analysed. All patients gave informed consent for the procedure and for data collection, according to the regulations of the respective local ethics committees.
PATIENT SELECTION
All patients deemed candidates for transcatheter aortic valve implantation (TAVI) by the local Heart Team and treated with the Allegra valve2 were included in this study. Endpoints were defined according to the VARC-2 criteria3.
STATISTICAL ANALYSIS
Statistical analyses were performed using SPSS, Version 23 (IBM Corp., Armonk, NY, USA). Continuous values were expressed as mean and standard deviation (SD). Categorical values were expressed as proportions of the total. Between-group comparisons were performed using a paired Student’s t-test, or Wilcoxon test. A two-tailed p-value of <0.05 was considered statistically significant.
DEVICE SPECIFICATIONS
The Allegra valve has a self-expanding nitinol stent frame with a supra-annular bovine pericardial valve. The specific design of the stent frame with variable radial forces along the stent, a concave stent shape and tip deflection during diastole aims at removing leaflet stress by up to 40% (according to the manufacturer’s preclinical study data), which may potentially enhance durability.
The valve is currently available in three different sizes (23 mm, 27 mm, and 31 mm), which are all delivered through a commercially available 18 Fr sheath. A sealing skirt of 12 mm at the ventricular part of the valve minimises the risk of paravalvular leaks.
The handle of the delivery system allows a stepwise, controlled release of the valve with stable haemodynamics throughout deployment without any flow obstruction. By repetitively squeezing the handle, the middle part of the valve stent starts bulging, allowing the leaflet tips to move within the stent frame (“Permaflow”). The lower marker is then aligned with the aortic annulus at the non-coronary cusp and tension on the delivery system is released (no forward push). The entire ventricular part of the valve is quickly deployed by squeezing the release knob. No rapid pacing is required; however, fast pacing at a rate of 100-140/min may be utilised if a large pulse pressure is present. At this stage, the valve is potentially fully retrievable by externalising the valve through the sheath and reinserting after re-crimping.
Once a satisfactory position is confirmed, the aortic part of the valve stent frame is released by using the handle to complete implantation.
PROCEDURE
The procedures were performed under local or general anaesthesia, according to the local institutional practice.
POST-PROCEDURAL FOLLOW-UP
Dual antiplatelet therapy with lifelong acetylsalicylic acid and three to six months of clopidogrel was routine, unless the patient had an indication for oral anticoagulation. Transthoracic echocardiography was performed before discharge and at 30 days.
Results
PATIENT RESULTS
Fifty-nine patients (mean age 78±8 years, 37% male, mean STS-PROM score 5.2±4.2) were included.
PROCEDURAL OUTCOMES
Balloon predilatation was performed in 82% of the cases. Post-dilatation was performed in 36% of patients due to malapposition, excessive paravalvular leak (PVL) or both. Device success was 95% and 30-day mortality was 1.7%. No stroke or major/life-threatening bleeding occurred. The rate of major vascular complications was 3.4% (Table 1).
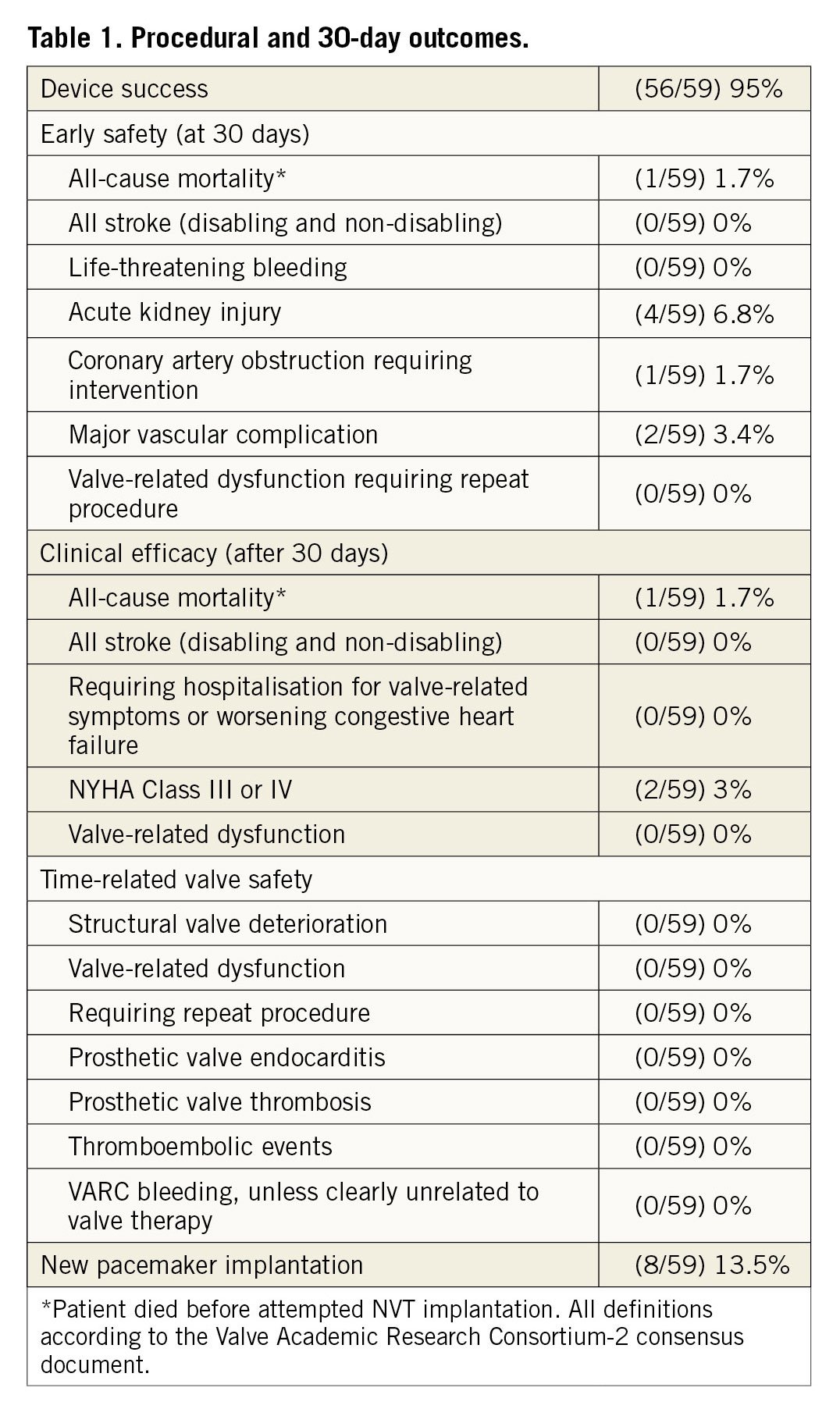
There was one periprocedural death. A polymorbid patient with severely reduced right ventricular function suffered from severe hypotension requiring mechanical resuscitation after insertion of the delivery sheath. Association with severe bradycardia made severe bleeding improbable. Despite fast and successful implantation of the Allegra valve, the patient did not recover and died in the catheterisation laboratory.
Acute kidney injury occurred in four patients (6.8%) and resolved in all patients before discharge.
The pacemaker implantation rate was 13.5% at 30 days.
VALVE PERFORMANCE RESULTS
Haemodynamic outcomes showed a mean gradient of 7.2±3.5 mmHg with an effective orifice area (EOA) of 2.06±0.3 cm2. Valve performance remained stable at 30 days. More than mild paravalvular leak was present in 5.1% of patients before discharge and 3.4% at 30 days.
PATIENT STATUS
At baseline, most patients suffered from NYHA Class III or IV symptoms. A significant improvement occurred at discharge and at 30 days (p<0.01). At 30 days >95% of patients were in NYHA Class I or II (Figure 1).
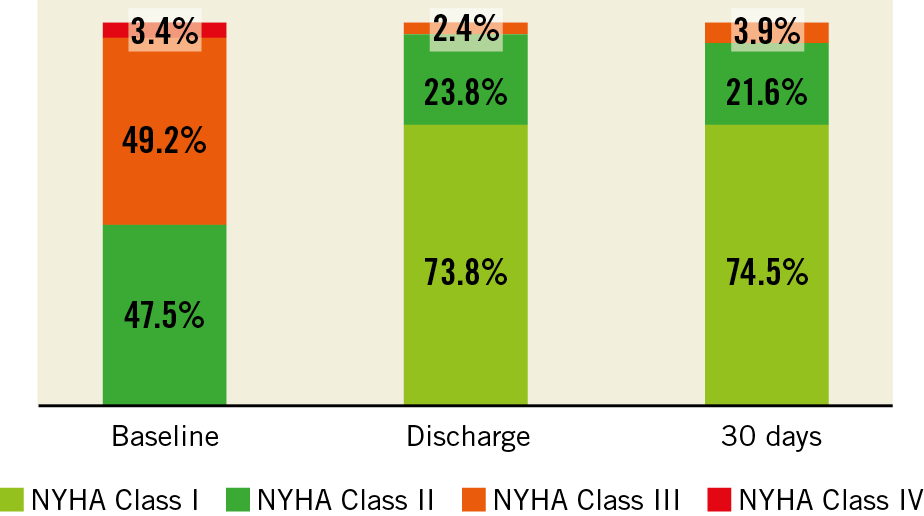
Figure 1. Functional capacity - NYHA grade at baseline, discharge and 30 days. Baseline vs. Discharge, p<0.01; Baseline vs. 30 days, p<0.01; Discharge vs. 30 days, p=NS.
Discussion
This multicentre European registry demonstrates good short-term haemodynamic outcomes with the novel Allegra transcatheter heart valve with low transvalvular gradients, a large EOA (>2 cm2) and low PVL rates (more than mild PVL in 3.4% of patients). Device success was 95%, comparable with other valves on the market.
In summary, in our real-world experience, the Allegra yielded good short-term outcomes.
Limitations
The current study is observational and limited by the low patient numbers. Larger studies are needed.
Conclusion
The Allegra transcatheter, self-expanding valve is safe and effective in the treatment of high- and intermediate-risk patients with severe aortic stenosis.
|
Impact on daily practice Our results show that transcatheter aortic valve implantation using the NVT Allegra valve results in good haemodynamic outcomes and a low complication rate. |
Conflict of interest statement
M. Taramasso is a consultant for Abbott Vascular and Boston Scientific. F. Nietlispach is a consultant for Edwards Lifesciences, Medtronic and Abbott Vascular. E. Molina is a consultant for New Valve Technology. R. Moreno is a consultant for New Valve Technology. S. Toggweiler is a consultant for Boston Scientific and New Valve Technology. The other authors have no conflicts of interest to declare.

