
Abstract
Aims: The aim of this study was to investigate the procedural and short-term outcomes of transcatheter aortic valve implantation (TAVI) with the Portico™ self-expanding, resheathable TAVI system from an ongoing long-term multicentre study.
Methods and results: A total of 941 patients (82.4±5.9 years, 65.7% female, STS score 5.8±4.9%) with severe symptomatic aortic stenosis underwent TAVI using the Portico bioprosthesis. Patients were clinically and echocardiographically assessed at implantation, discharge and 30 days post TAVI. An independent CEC and core laboratory adjudicated adverse events (according to VARC-2) and follow-up echocardiograms, respectively. Implantation was successful in 96.0% of patients. Thirty-day all-cause, cardiovascular mortality and disabling stroke rates were 2.7%, 2.4% and 1.6%, respectively. Major vascular complications and life-threatening bleeding occurred in 5.5% and 3.1% of patients, respectively. A new pacemaker was implanted in 18.7% of patients. Aortic valve area (0.70±0.33 cm2 vs. 1.79±0.48 cm2) and transvalvular gradient (49.7±15.3 mmHg vs. 8.6±3.9 mmHg) improved significantly. The 30-day rate of moderate or higher paravalvular leak (PVL) was 3.9%.
Conclusions: The Portico TAVI system allows safe and effective treatment of aortic stenosis in patients at increased surgical risk. At 30 days, mortality was low, and good haemodynamic performance was indicated by low transvalvular gradient and a low rate of moderate or higher PVL.
Abbreviations
AKI: acute kidney injury
AS: aortic stenosis
AVA: aortic valve area
CEC: clinical events committee
CT: computed tomography
NOAF: new-onset atrial fibrillation
PVL: paravalvular leak
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) is a recommended treatment for patients with symptomatic aortic valve stenosis (AS) who are at increased surgical risk1,2, taking into account other factors such as patient frailty, comorbidities and patient preferences. Several randomised controlled trials have shown favourable results with TAVI when compared with surgical valve replacement in high-risk and intermediate-risk patients3-6. Various outcomes, such as the occurrence of paravalvular leak (PVL) and the risk of conduction disturbances requiring implantation of a permanent new pacemaker, depend on optimal positioning of the bioprosthesis. The self-expanding Portico™ transcatheter bioprosthetic aortic valve (St. Jude Medical [now Abbott], St. Paul, MN, USA) has resheathing capabilities allowing valve repositioning during the implantation procedure7. This study reports on the procedural and 30-day outcomes of an ongoing multicentre evaluation, assessing the long-term (five-year) clinical outcomes of this device.
Methods
STUDY DESIGN
The ongoing, international, multicentre PORTICO-1 study is a prospective, single-arm, non-randomised clinical investigation (ClinicalTrials.gov: NCT01802788). The objective of the study is to assess long-term clinical outcomes of the Portico valve for treatment of severe, symptomatic AS. The study includes 61 centres in Europe (n=43), Canada (n=8) and Australia (n=10). Study centres functioned in compliance with the Declaration of Helsinki and approvals from ethics committees and local authorities were obtained. All patients provided written informed consent prior to participation. Abbott (formerly St. Jude Medical) sponsored the study. The initial experience of the participating centres with the Portico TAVI system at 30 days post TAVI, including evaluation of echocardiograms and adverse events, is presented here.
PATIENTS AND RISK ASSESSMENT
Patients with echocardiographically confirmed severe AS were eligible for participation. Echocardiographic criteria included an aortic valve area (AVA) ≤1.0 cm2 or indexed effective orifice area ≤0.6 cm2/m2 AND a mean aortic gradient >40 mmHg or jet velocity >4.0 m/s or Doppler velocity index <0.25. Patients were referred by the local Heart Teams based on their high risk for surgery as demonstrated by the Society of Thoracic Surgeons Predicted Risk of Operative Mortality (STS-PROM) and logistic European System for Cardiac Operative Risk Evaluation scores. A clinical judgement of the Heart Team, based on the individual risk profile (e.g., frailty and comorbidities not captured by STS score), was also accepted. Key exclusion criteria included a non-tricuspid aortic valve, the presence of a prosthetic aortic valve (valve-in-valve procedures), or the need for concomitant structural heart procedure. All inclusion/exclusion criteria are shown in Table 1.

VALVE IMPLANTATION AND FOLLOW-UP
All patients underwent transfemoral TAVI using the Portico TAVI system (available in sizes 23, 25, 27 and 29 mm), which has been described in detail elsewhere7. Key characteristics include a large-cell, self-expanding nitinol frame, bovine pericardial leaflets functioning at the annular level, resheathing and repositioning capabilities, and a transfemoral delivery system compatible with expandable sheaths such as the Portico Solo™ device (St. Jude Medical [now Abbott]) for an ultra-low insertion profile (11 Fr). Sheathless implantation occurred in some cases.
Implanting physicians were trained according to the sponsor. Based on early physician feedback, and data analysis of initial studies with the Portico valve, the training programme was updated to include a greater emphasis on using computed tomography (CT) for sizing, the value of balloon predilatation, the importance of targeting an implant depth of 3-4 mm, and guidelines for post-dilatation. As a result, valve sizing was preferably based on preprocedural imaging with multislice CT. Balloon predilatation and valve positioning without rapid pacing were recommended. Valve resheathing and repositioning was performed if indicated. The valve was released after angiographic confirmation of the appropriate implant position. Post-dilatation was performed at the discretion of the operator to improve sealing and full device expansion at the annulus if aortic insufficiency or valve underexpansion was present. Post-dilatation was performed under rapid pacing to prevent valve dislocation.
Post-procedural antithrombotic therapy was at the physician’s discretion.
Patients are followed at 30 days, one year and annually up to five years post implant. Patients without an implant attempt (i.e., delivery system not introduced into the body) were followed until 30 days after the procedure but are not included in this report. Echocardiographic evaluations are performed at baseline, implantation, pre-discharge, 30 days, one year and annually up to five years post implant.
STUDY ENDPOINTS, ASSESSMENTS AND DEFINITIONS
The primary endpoint of this study is all-cause mortality at one year. Secondary clinical endpoints include cardiovascular mortality, myocardial infarction, stroke, acute kidney injury (AKI), access-related complications and bleeding at 30 days. Additional 30-day outcomes include new or worsened conduction disturbances and cardiac arrhythmias, the need for new pacemaker implantation, new-onset atrial fibrillation (NOAF) and NYHA functional classification. An independent clinical events committee (CEC) adjudicated all safety endpoints according to VARC-2 guidelines8.
Implant success was defined as successful vascular access, delivery and deployment, a single functioning valve in the proper position and successful retrieval of the delivery system. Total procedure time was defined as access to closure of the vascular access site.
Thirty-day echocardiographic assessments were evaluated by an independent echocardiographic core laboratory (MedStar Health Research Institute, Washington DC, USA). Total aortic regurgitation (AR) severity with paravalvular and transvalvular components was classified into four classes (none/trace, mild, moderate, severe) following VARC-2 and American Society of Echocardiography criteria9. Prosthetic valve haemodynamics were analysed according to American Society of Echocardiography guidelines10. Implantation depth was measured angiographically and was site-reported.
STATISTICAL ANALYSIS
Continuous variables were summarised by mean±standard deviation (SD). Categorical variables were summarised using frequencies and percentages. Paired Student’s t-tests (echocardiographic data, 6-min walk test) and the Wilcoxon signed-rank test (NYHA functional class) were used to compare outcomes at 30 days relative to baseline. Evaluation of all adverse events was based on CEC-adjudicated outcomes. Comparisons of adverse event and PVL rates across valve sizes were performed using Fisher’s exact test. Statistical significance was set at p<0.05. All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, USA).
Results
PATIENTS
Between April 2013 and June 2017, implantation of a Portico bioprosthetic aortic valve was attempted in 941 patients (Table 2). The patients’ mean age was 82.4±5.9 years, 65.7% were female and 64% were severely symptomatic (NYHA Class III/ IV). Mean STS score was 5.8±4.9% (median: 4.3%). Coronary artery disease was present in 50.3% of the patients, atrial fibrillation in 17.2%, and moderate/severe mitral regurgitation in 25.2%.
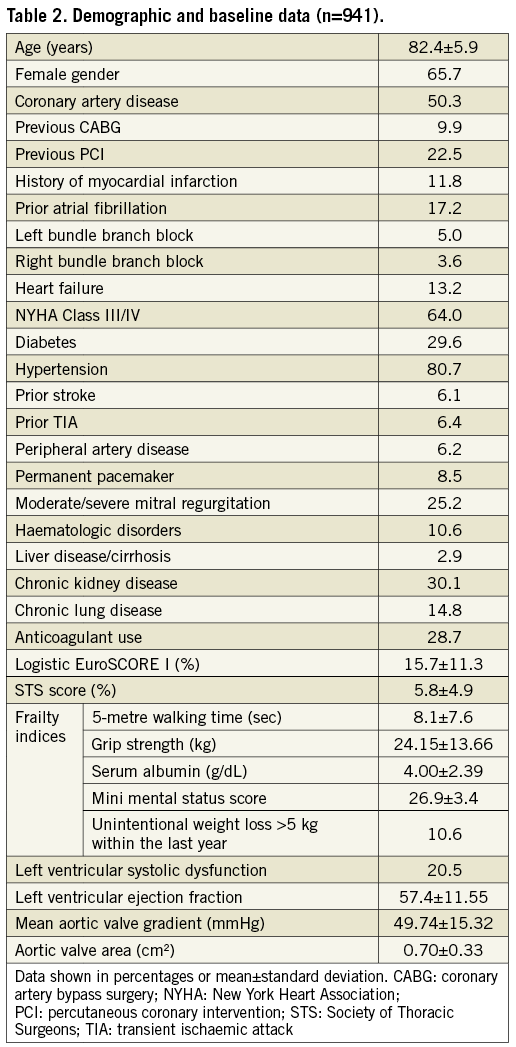
PROCEDURAL OUTCOMES
Procedural characteristics and outcomes are provided in Table 3. Predilatation and resheathing/repositioning were performed in 88.9% and 41.4% of patients, respectively.
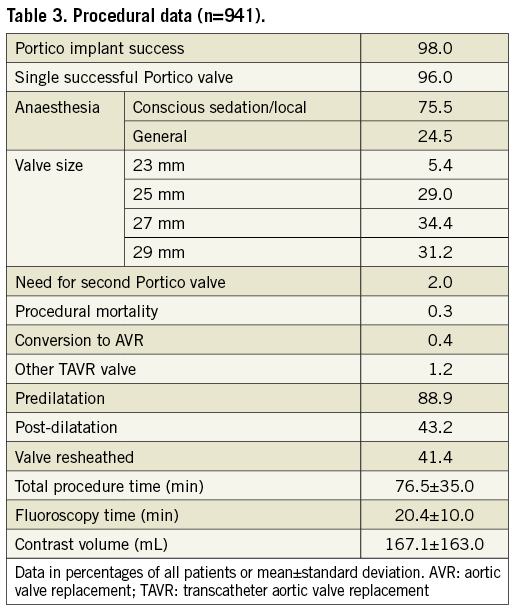
In total, 922/941 TAVI procedures were completed with a functioning valve (98%), including 903 (96%) single valve implants and 19 cases (2%) utilising two Portico valves. Nineteen unsuccessful implantations were related to procedural mortality (n=3, 0.3%), conversion to AVR (n=4, 0.4%) and implantation of an alternative commercial TAVR device due to annulus size, Portico migration upon release, or TAV-in-TAV placement to reduce PVL (n=12, 1.2%). Procedural deaths were due to dissection of the right coronary artery ostium, aortic dissection and aortic perforation (one case [0.1% occurrence] of each).
30-DAY OUTCOMES
Thirty-day visits were recorded from 828 patients. Of the 110 patients with no documented 30-day visit, 22 died after the procedure, 24 were withdrawn and 64 missed the 30-day visit. However, 52 had a subsequent visit or were followed for a reported adverse event.
Thirty-day post-TAVI outcomes are summarised in Table 4. Thirty-day all-cause mortality was 2.7% (25 patients), including three procedural deaths, 16 post-procedural in-hospital deaths and six deaths after discharge. Cardiovascular mortality at 30 days was 2.4%, and most frequently related to post-procedural complications, cardiac or aortic perforation, coronary obstruction or occlusion and sudden death of unknown cause.
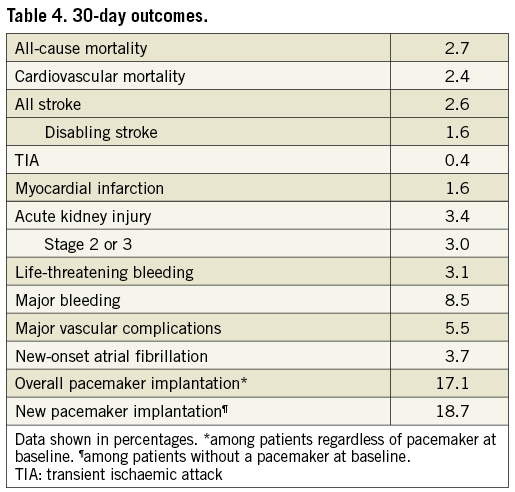
Disabling stroke occurred in 15 patients (1.6%) and 32 patients (3.4%) had acute kidney injury (AKI), requiring interim haemodialysis in 10 cases (1.1%). As adjudicated, 29 patients (3.1%) had life-threatening bleeding. Major vascular complications occurred in 5.5% of the patients and were mostly access-related. Transfusion was required in 24 cases (2.6%).
A permanent pacemaker was implanted in 161 patients, representing 17.1% of all patients regardless of pacemaker status at baseline and 18.7% of patients with no prior pacemaker. Pacemaker implantation was associated with greater implant depth (depth relative to the annulus: 6.2±2.7 mm vs. 5.2±2.5 mm, p<0.0001) and higher prevalence of conduction disorder at baseline (21.7% vs. 11.1%, p=0.0003). Regarding predilatation or post-dilatation, the balloon-to-annulus ratio and the balloon-to-left ventricular outflow tract ratio were not significantly different between the two groups.
The AVA increased from 0.70±0.33 cm2 at baseline to 1.79±0.50 cm2 at 30 days (p<0.0001) (Figure 1). At 30 days post TAVI, the mean aortic transvalvular gradient was reduced to 8.6±3.9 mmHg, compared with 49.7±15.3 mmHg at baseline (p<0.0001). At 30 days post TAVI, moderate or higher PVL was observed in 3.9% of the patients (all moderate; no cases of severe PVL were observed). Moderate or higher PVL, compared to mild or lower PVL, was associated with a deeper implant (site-reported angiographic measurement) and more frequent post-dilatation, while the rate of predilatation was the same in both groups. The lowest and highest rates of moderate or higher PVL were observed with 27 mm valves (1.7% of valves) and 25 mm valves (6.5%), respectively. Sites performing >15 procedures achieved a lower rate of moderate or higher PVL than sites with fewer procedures (2.6% versus 7.1%, p=0.0085). This was driven by a substantial reduction in the rate of moderate or higher PVL from 5.9% in the first 15 procedures to 2.1% in the remaining, later cases.
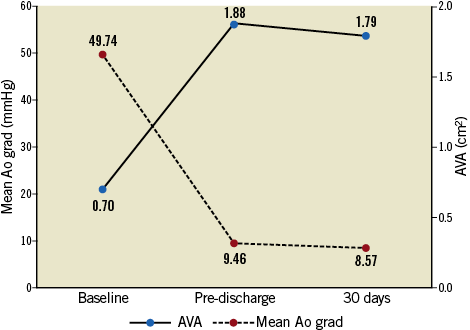
Figure 1. Aortic valve area (AVA) and mean aortic valve gradient.
TAVI was associated with improvement in NYHA functional class (p<0.0001), with 13.1% of patients in NYHA Class III/IV at 30 days, versus 64% at baseline (Figure 2).
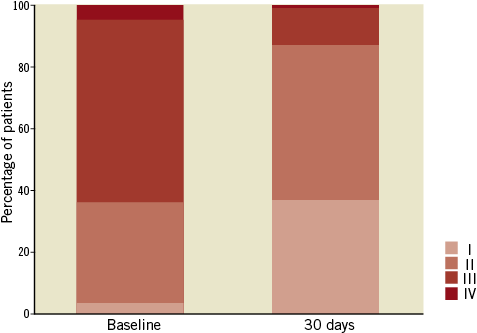
Figure 2. NYHA class at baseline and at 30 days post TAVI.
No significant differences were found among valve sizes with regard to adjudicated adverse events and the rate of pacemaker implantation.
Discussion
This study reports 30-day outcomes of a large, multicentre real-world cohort of patients with severe, symptomatic AS undergoing TAVI with the Portico bioprosthetic aortic valve. While TAVI was initially introduced as a less invasive alternative to surgical valve replacement for patients with high surgical risk, lower-risk patients are increasingly considered for TAVI. With a mean STS score of 5.8%, the present study follows this trend. However, other factors not included in the STS assessment may have contributed to a higher surgical risk. Most importantly, frailty and comorbidities were accounted for by the local Heart Teams in assessing the individual surgical risk profile of patients considered for aortic valve replacement. Furthermore, the proportion of subjects with a logistic EuroSCORE I >10% was 62%.
A single Portico valve was successfully implanted in 96.0% of patients. This outcome compares well with results reported from other studies11,12 using this device, considering it was achieved by 61 centres worldwide with variable experience and patient load. Immediate, procedural mortality (0.3%) as well as all-cause mortality at 30 days post TAVI (2.7%) were relatively low and comparable with outcomes reported from other TAVI studies with either the Portico or other transcatheter bioprosthetic aortic valves5,11-14. Specifically, 30-day mortality in this study was similar to other studies in populations of similar risk6,11,15-18. Nevertheless, direct comparison between cohorts, even with similar STS score, remains difficult, given other risk factors and conditions that are not captured by the STS score. Within the 30-day post-TAVI period, disabling stroke occurred at a rate of 1.6%, which is within the range of disabling stroke rates reported by other studies on the Portico device11,12 and from other devices13,14.
Relatively low rates of permanent pacemaker implantation have been reported from other series with the Portico valve11,12. In this large cohort, a new permanent pacemaker was implanted in 18.7% of patients without prior pacemaker, which is slightly more than in earlier, smaller series with this device11,12. This may be partially due to the conduction disorders at baseline, which were significantly more frequent in patients who received a new pacemaker compared to those without. Nevertheless, the achieved rate of pacemaker implantation in patients at risk is similar to other self-expanding and balloon-expandable prosthetic valves19,20 (CoreValve® [Medtronic, Minneapolis, MN, USA]: 28.7%21, Evolut™ R [Medtronic]: 19.3%14, SAPIEN 3 [Edwards Lifesciences, Irvine, CA, USA]: 19.7%3).
In this study, an independent core laboratory provided consistent and unbiased evaluation of echocardiograms. Thirty-day assessment showed significant and clinically relevant improvements from baseline in AVA and aortic transvalvular gradient. During the first part of this study, only the two smaller size Portico valves were available. Therefore, an even larger mean AVA may be expected in a contemporary setting with access to all valve sizes. At 30 days, PVL was moderate in 3.9% of patients and no severe PVL was observed. This outcome is comparable to outcomes reported from other contemporary self-expanding aortic valves, such as the CoreValve (11.4%)21, Evolut R (5.3%)14, 1.9% at discharge16, or the ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) (4.8%)22 or from balloon-expandable bioprostheses, such as the SAPIEN 3 (3.4%)13 or the LOTUS™ valve (Boston Scientific) (0.5%)18. In 60 patients implanted with the Evolut™ PRO device (Medtronic), no moderate or higher PVL was seen23. Results from this study suggest a learning curve effect with respect to PVL. Sites that enrolled >15 patients achieved improved outcomes regarding PVL at 30 days post TAVI. Moreover, the low rate of moderate or higher PVL was associated with frequent predilatation (88.9%) and post-dilatation in 43.2% of the procedures. While predilatation may be less common in current TAVI practices with other valves, these results suggest that it may play an important role in avoiding PVL after implantation of the Portico valve. Given the conformability of large-cell self-expanding and resheathable stents, predilatation facilitates a more gradual and uniform stent deployment. As this may allow better accommodation of the stent within the aortic annulus and of calcific nodules within the stent cells, predilatation was recommended as part of the training programme for this study. In addition, valve resheathing and repositioning, applied in 41.3% of the procedures, may have facilitated a more optimal valve placement and prevention of PVL. Overall, applying predilatation and gradual valve deployment appear to be important aspects of the learning curve for operators gaining experience with the Portico TAVI system. The lack of difference found in the use of predilatation between patients with and without more-than-moderate PVL was most likely due to the frequent use of predilatation in this study (88.9%).
No significant differences in rates of adverse events and pacemaker implantation were found between the valve sizes.
Recently, a multicentre study including 222 patients implanted with the Portico bioprosthesis reported low one-year mortality and stroke rates24. Ongoing follow-up of the present study will show whether these results can be confirmed by data obtained from a larger population. Longer-term follow-up will also assess the benefits of the large cell dimensions of the stent frame of this device regarding coronary access for percutaneous interventions after TAVI.
Limitations
This study involved non-random selection of patients to be treated with the Portico TAVI system. The study enrolment occurred over an extended four-year period, beginning in 2013 after CE mark of the Portico 23 mm valve. Naturally, enrolment was slow until the full family of valve sizes was commercially available in September 2015. Once all sizes were available, 61 sites were activated and the remaining population (85%) was promptly enrolled. Data were obtained from centres with variable experience and patient load, providing a cross-section of centres performing TAVI, and included the initial learning curve in most institutions.
Conclusions
The Portico TAVI system allows safe aortic valve implantation in patients with severe, symptomatic AS. Short-term clinical outcomes are characterised by low all-cause and cardiovascular mortality and good haemodynamic performance, including low transvalvular gradients and a low incidence of moderate or higher PVL.
| Impact on daily practice Procedural and 30-day outcomes show that the Portico TAVI system is safe to implant and provides acceptable haemodynamic performance in the short term, including low transvalvular gradients and low rates of moderate or higher paravalvular leak. |
Funding
This study was funded by Abbott.
Conflict of interest statement
L. Søndergaard has received consultant fees and an institutional research grant from Abbott (formerly SJM). U. Schäfer, S. Fichtlscherer and F. Maisano are proctors for Abbott Vascular and have received travel/speaker’s honoraria and grant support. J. Rodés-Cabau has received institutional research grants from Abbott. A. Linke and G. Fontana have received speaker honoraria as consultants and grant support from Abbott. R. Makkar and S. Worthley have received consultancy fees from Abbott. F. Asch is Director of MedStar Health with institutional contracts from Abbott.

