
Transcatheter aortic valve implantation (TAVI) is currently moving towards a lower-risk population1. However, in order to aim for TAVI non-inferiority to SAVR in the setting of a low-risk population, two cornerstones must be established: 1) optimal procedural results (a low procedural mortality, incidence of stroke, significant paravalvular leak, new permanent pacemaker implantation and major vascular complications); 2) good long-term follow-up (adequate prosthesis durability; easy re-access for the coronary arteries; feasibility of TAVI-in-TAVI).
How to obtain optimal procedural results
In this issue of EuroIntervention, Millan-Iturbe et al2 report the 30-day and one-year clinical outcomes of 216 patients treated with the Portico™ valve (St. Jude Medical [now Abbott Vascular], St. Paul, MN, USA).
Of note, the mean STS score of the study population was 4.3% and 128 patients (59%) had an STS score <4%, thus becoming the largest cohort of low-risk all-comer patients implanted with the Portico valve.
The overall procedural success was high with device success achieved in 94.4% of the procedures. The rate of permanent pacemaker implantation was 15.8%, while major vascular complications occurred in 6.0% of the patients; the overall rate of stroke was low (0.5%). At 30-day follow-up, aortic valve area increased from 0.7±0.2 cm2 at baseline to 1.9±0.4 cm2 and the mean aortic valve gradient decreased from 46.5±14.1 mmHg at baseline to 7.4±3.8 mmHg (p<0.01) (Figure 1).
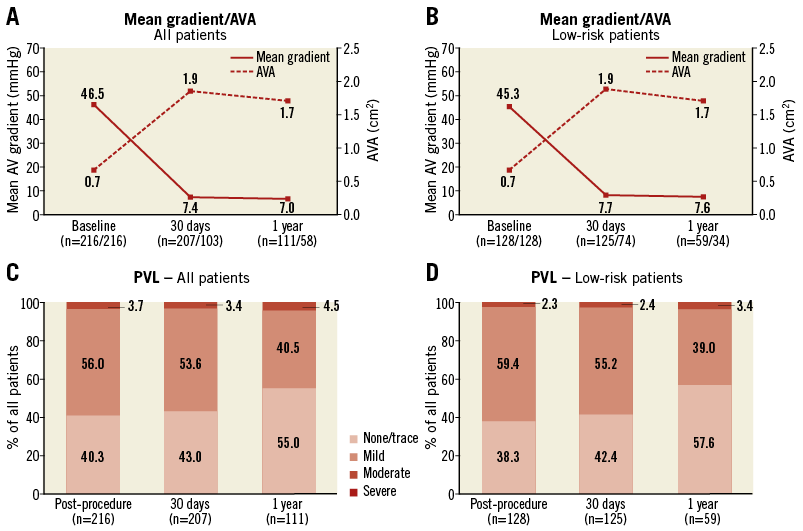
Figure 1. Valve function. Mean transvalvular gradient and AVA at baseline, 30 days and one year after TAVI for the entire study cohort (A) and the low surgical risk group (B). Paravalvular leak (PVL) immediately post procedure, at 30 days and one year after TAVI for the entire study cohort (C) and the low surgical risk group (D), as assessed by transthoracic echocardiography. Numbers of patients included in the assessment are indicated along the horizontal axis. AVA: aortic valve area; PVL: paravalvular leak. Reprinted from Millan-Iturbe et al2 with permission from Europa Digital & Publishing.
Two aspects should be underlined: 1) procedural results in this low-risk population were good, with a high device success rate (97.7%), no procedural mortality, a low rate of moderate-to-severe paravalvular leak (2.3%); 2) a trend towards an improvement in composite clinical outcomes which appears to be experience-related, with lower rates of valve embolisation, more-than-mild paravalvular leak and pacemaker implantation observed in later procedures.
These results are in line with currently available data on a low-risk TAVI population: in the NOTION trial (Nordic Aortic Valve Intervention Trial)3, 280 low-risk patients were randomised to TAVI (first-generation self-expanding CoreValve® [Medtronic, Minneapolis, MN, USA]) versus SAVR. After one year, no significant differences were found between the two groups in terms of death from any cause, stroke, or myocardial infarction. TAVI procedures were highly successful (97.9%), with 30-day all-cause death comparable to surgery (2.1% vs. 3.7%, p=0.43), a low incidence of neurological events (2.8%) and major vascular complication (5.6%); permanent pacemaker implantation was higher in the TAVI group (34.1% vs. 1.6%, p<0.001).
Millan-Iturbe et al have shown how “the first cornerstone” can be achieved with Portico implantation using an optimal procedural strategy together with an optimal implantation technique.
CHOICE OF THE BEST PERCUTANEOUS ACCESS
In order to minimise the incidence of major vascular complications, a meticulous evaluation of the vascular access should be carried out. Recently, alternative routes in TAVI (transaxillary/trans-subclavian, transcaval)4,5 have shown reasonable clinical outcomes. In the population of Millan-Iturbe et al, a low incidence of major vascular complications was reported (6.0%). Interestingly, the use of other than transfemoral access was non-negligible (5.6% subclavian, 3.2% transcaval).
The Portico requires an 18 Fr sheath for the 23 and 25 mm prostheses and an 19 Fr sheath for the 27 and 29 mm prostheses. The Ultimum™ introducer sheath (St. Jude Medical) and a Check-Flo® Performer™ sheath (Cook Medical, Bloomington, IN, USA) are commonly utilised: the Ultimum has a smaller external diameter, but is less braided than the Cook; thus, the Ultimum sheath may be easier to introduce in borderline vessel sizes, but may kink in tortuous vessels6. A sheathless approach with the Portico valve has been described7: it was used in the Millan-Iturbe series in case of small vessel diameter (4.5-5.0 mm) (10.6% of the overall transfemoral population) with good outcomes.
THE IMPORTANCE OF A PRECISE DEVICE IMPLANTATION
In order to decrease the risk of paravalvular leak and new pacemaker implantation, predilatation might be required, given the relatively low radial force of the current generation of self-expanding valves. The ideal depth of implantation is represented by the frame’s inflow edge placed 3-4 mm below the aortic annulus. In case of a relatively deep implant (>9-10 mm below the annulus), partially re-sheathing and repositioning the valve instead of pulling the system to a higher position is preferred6. Valve underexpansion or malapposition should be corrected with balloon post-dilatation.
How to reach good midterm to long-term follow-up
In order to achieve the second cornerstone, with good clinical outcome after TAVI, the prosthesis must guarantee good durability and should not interfere with coronary ostia cannulation.
Clinical valve thrombosis incidence after TAVI has been shown to be around 2.8% and to be more frequent in balloon-expandable valves8. The incidence of subclinical TAVI thrombosis appears higher but its impact on valve durability is still unknown9.
Limited data are available about TAVI prosthesis durability. Holy et al reported the outcome of 152 patients implanted with the CoreValve: at six-year follow-up the mean echocardiographic follow-up was excellent, with a rate of valve failure of 7.9% at eight years10. Although those data appear favourable, larger series and longer follow-up are required because a younger population should theoretically lead to faster prosthesis degeneration.
Given the intra-annular position and the larger cell design of the Portico prosthesis, it could theoretically facilitate coronary ostia cannulation compared with other self-expanding prostheses.
Conclusions
The new-generation Portico valve is associated with promising periprocedural results. Longer follow-up is still needed in order to extend its applicability routinely to a younger and lower-risk population.
Conflict of interest statement
The authors have no conflicts of interest to declare.

