Abstract
Background: The Navitor transcatheter heart valve (THV) is a self-expanding valve, with an intra-annular leaflet position and an outer cuff intended to reduce paravalvular leak (PVL).
Aims: The aim of the PORTICO NG Study is to assess the safety and performance of the Navitor THV in patients with symptomatic, severe aortic stenosis who are at high or extreme surgical risk.
Methods: PORTICO NG is a prospective, multicentre, global, single-arm, investigational study with follow-up at 30 days, 1 year, and annually up to 5 years. The primary endpoints are all-cause mortality and moderate or greater PVL at 30 days. Valve Academic Research Consortium-2 events and valve performance are assessed by an independent clinical events committee and echocardiographic core laboratory.
Results: A total of 120 high- or extreme-risk subjects (age 83.5±5.4 years; 58.3% female; Society of Thoracic Surgeons score 4.0±2.0%) were enrolled in the European conformity (CE) mark cohort. Procedural success was high at 97.5%. At 30 days, the rate of all-cause mortality was 0%, and no subjects had moderate or greater PVL. The rate of disabling stroke was 0.8%, life-threatening bleeding was 2.5%, stage 3 acute kidney injury 0%, major vascular complications 0.8%, and new pacemaker implantation 15.0%. At 1 year, the rates of all-cause mortality and disabling stroke were 4.2% and 0.8%, respectively. The rate of moderate PVL was 1.0% at 1 year. Haemodynamic performance with a mean gradient of 7.5±3.2 mmHg and effective orifice area of 1.9±0.4 cm2 was sustained up to 1 year.
Conclusions: The PORTICO NG Study demonstrates low rates of adverse events and PVL up to 1 year in patients at high or extreme surgical risk, confirming the safety and efficacy of the Navitor THV system.
Introduction
Transcatheter aortic valve implantation (TAVI) is an effective therapy to treat patients with symptomatic, severe aortic stenosis and is recommended in elderly patients across all surgical risk strata12. As TAVI is increasingly used in younger patients with a longer life expectancy, the impact of valve design on, e.g., haemodynamic performance, paravalvular leak (PVL), and future coronary access is crucial for proper lifetime management.
PVL remains a common concern, as it occurs more frequently following TAVI compared to surgical aortic valve replacement (SAVR), regardless of valve type and patient surgical risk34567. Additionally, as PVL is associated with increased mortality and may be associated with a need for reintervention, special attention is given to the design of transcatheter heart valves (THV). Thus, next-generation THV devices include modifications, such as an outer pericardial wrap or synthetic sealing skirt, to mitigate the risk of PVL.
The Navitor THV (Abbott) is an iteration of the Portico THV and includes an outer fabric cuff, known as the NaviSeal cuff, to actively reduce the risk of paravalvular leak (Central illustration). Together with the FlexNav delivery system (Abbott), the Navitor THV system is optimised to provide favourable clinical outcomes while maintaining enhanced deliverability and ease of use.
The PORTICO NG Study aimed to investigate the safety and effectiveness of the Navitor THV System in subjects who are at high or extreme surgical risk. The current report describes the first 120 subjects (i.e., the CE mark cohort) treated with the Navitor THV system up to 1 year.
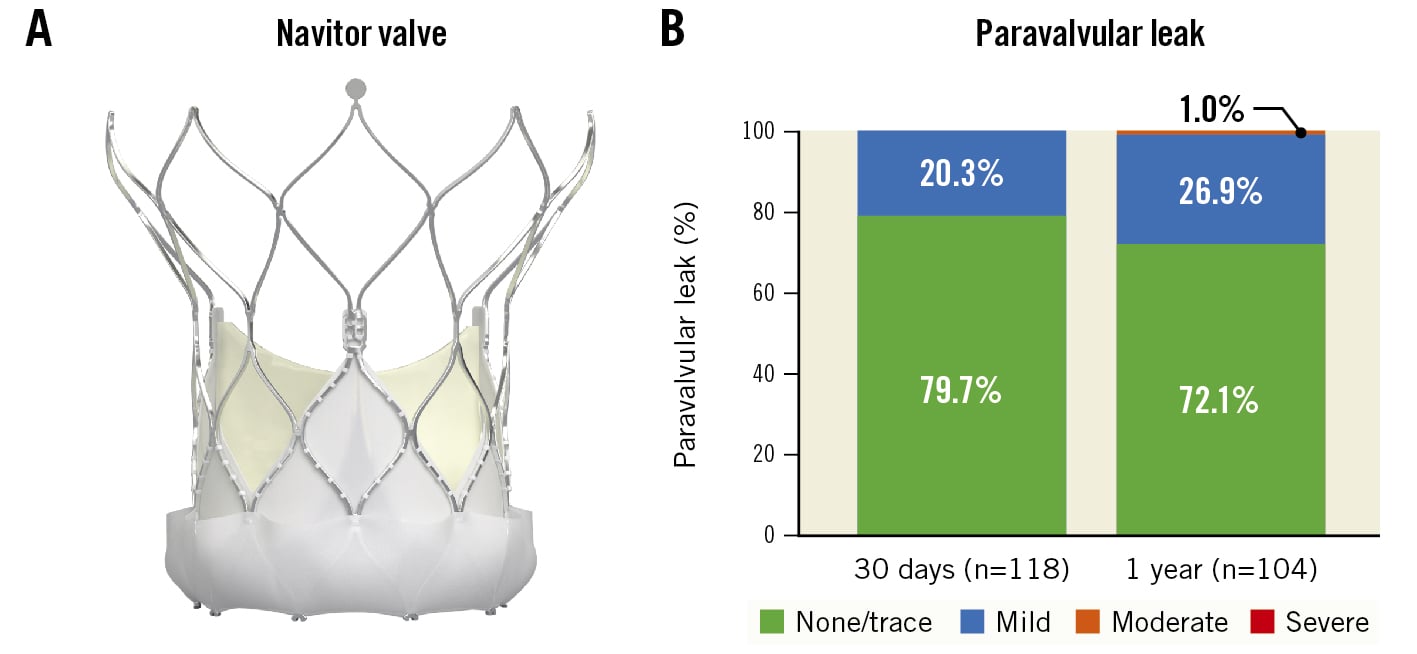
Central illustration. The Navitor valve is optimised to increase valve sealing and reduce paravalvular leak. A) The Navitor valve is a repositionable, self-expanding, intra-annular valve with large stent cells to preserve coronary access for future intervention. The active outer NaviSeal cuff reduces the risk of paravalvular leak. The target implant depth is 3 mm below the native aortic annulus. B) Degree of paravalvular leak at 30 days and 1 year. No subjects experienced moderate or greater paravalvular leak at 30 days, and only 1 subject (1.0%) experienced moderate paravalvular leak at 1 year
Methods
Study design
The PORTICO NG Study is a prospective, multicentre, global, single-arm, investigational study conducted at sites in the US, Europe and Australia (Supplementary Table 1). The study population included subjects with severe, symptomatic aortic stenosis (AS) who were considered to be at high or extreme surgical risk. Subjects underwent TAVI via a transfemoral or alternative access route using the Navitor THV and the FlexNav delivery system (DS), collectively known as the Navitor THV system. Study assessments were performed at baseline, discharge, 30 days, and 1 year. The annual follow-up, up to 5 years, is ongoing.
An independent clinical events committee (CEC) adjudicated all endpoint-related events according to the Valve Academic Research Consortium-2 (VARC-2) definitions, and an independent core laboratory (MedStar Health Research Institute) evaluated all echocardiographic data.
The institutional review board/ethics committee at each study site approved the study protocol prior to subject enrolment. All subjects provided written informed consent. The PORTICO NG â¨Study is registered at ClinicalTrials.gov: NCT04011722 and is sponsored by Abbott.
Study device
The Navitor THV is a repositionable, self-expanding valve, with a non-tapered stent to reduce interaction with the conduction system, large stent cells to facilitate coronary access for future intervention, and three bovine pericardial tissue leaflets positioned intra-annularly. The Navitor THV is available in four sizes (23, 25, 27 and 29 mm) covering an aortic annulus range from 19 to 27 mm; there is no change in valve sizing compared to the first-generation Portico valve. Key design features of the Navitor THV include the active NaviSeal cuff on the exterior portion of the stent to optimise valve sealing and reduce PVL, a more uniform chronic outward radial force across all valve sizes, and minor modifications to the stent design intended to minimise vessel trauma (Central illustration). The height of the NaviSeal cuff is 9 mm for the 23 and 25 mm valves and 10 mm for the 27 and 29 mm valves. To optimise valve sealing and minimise the risk of conduction disturbances post-implantation, the target implant depth with the Navitor THV is 3 mm. The Navitor THV is compatible with the FlexNav DS, designed to offer enhanced flexibility for deliverability and stable positioning during deployment. The 23 and 25 mm Navitor THVs can be implanted using the 14 Fr equivalent FlexNav DS in access vessels with a diameter ≥5.0 mm, and the 27 and 29 mm Navitor THVs can be implanted using the 15 Fr equivalent large FlexNav DS in diameters ≥5.5 mm.
Study procedures
Inclusion criteria were the presence of severe symptomatic aortic stenosis (AS) in subjects who were deemed at high or extreme risk by a Heart Team at each study site. Severe AS required documentation of the aortic valve area (AVA) of ≤1.0 cm2 (or the indexed effective orifice area [EOA] ≤0.6 cm2/m2) AND either a mean gradient ≥40 mmHg or peak jet velocity ≥4.0 m/s or Doppler velocity index ≤0.25. Subjects assessed with a New York Heart Association (NYHA) Functional Class of II or greater were considered symptomatic. Subjects were classified as high risk based on a Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score of ≥7% or if frailty indices and/or existing comorbidities not captured by STS scoring were also present. Subjects with factors precluding SAVR, based on the probability of death or serious morbidity exceeding 50% at 30 days, were classified as extreme risk.
All subjects were reviewed by an independent screening committee consisting of physician investigators to confirm eligibility, surgical risk, and anatomical suitability. Key imaging exclusion criteria included congenital unicuspid or bicuspid native valve anatomy or a non-calcified native aortic valve. Other key exclusion criteria included evidence of myocardial infarction, or any coronary or peripheral interventional procedure performed within 30 days prior to the index procedure, gastrointestinal bleeding preventing the use of antithrombotic therapy, blood dyscrasias, a pre-existing prosthetic heart valve, other implant in any valve position, or life expectancy of <1 year. All subjects were required to undergo multislice computed tomography (CT) prior to the procedure for proper device sizing and procedural planning.
Valve implantation was performed under general or local anaesthesia, with the access site prepared according to standard practice. Predilatation by balloon aortic valvuloplasty of the native aortic valve was recommended per the instructions for use (IFU). Use of the FlexNav DS integrated sheath alone or with an external introducer sheath to deploy the Navitor THV was at the operator’s discretion. Valve resheathing and repositioning were performed prior to full release (i.e., 80% deployment) if the implantation depth or positioning were suboptimal. If needed, the valve may be resheathed and repositioned up to two times prior to full release, per the IFU. Post-dilatation was performed at the discretion of the operator to improve sealing if clinically significant paravalvular leak or valve underexpansion were present. Post-procedural antithrombotic therapy was administered per the site’s standard of care.
Endpoints
The primary safety endpoint was all-cause mortality at 30 days, and the primary effectiveness endpoint was moderate or greater PVL at 30 days. A descriptive secondary endpoint evaluated the non-hierarchical composite safety endpoint of all-cause mortality, disabling stroke, life-threatening bleeding, stage 3 acute kidney injury (AKI), or major vascular complications at 30 days from the index procedure. Additional descriptive endpoints related to procedural success, valve performance, functional status and quality of life were assessed up to 30 days. Procedural success was defined as the correct positioning of a single Navitor THV in the annulus. Adverse events were defined using VARC-2 criteria. Echocardiographic assessments included the EOA, mean aortic transvalvular gradient, and paravalvular leak according to VARC-2 criteria. Functional status was evaluated using the six-minute walk test (6MWT) and NYHA Class. Quality of life was assessed using the Short Form-36 (SF-36) Health Survey score.
Statistical methods
Baseline characteristics, procedural outcomes and study endpoints were summarised using descriptive statistics. A paired t-test (echocardiographic data) and the Wilcoxon signed-rank test (NYHA Class) were used to compare outcomes at 30 days relative to baseline and/or discharge in subjects with available data. Statistical significance was indicated by a p-value <0.05. Statistical analyses were performed using SAS software version 9.4 (SAS Institute).
Results
Subjects
One hundred and twenty subjects underwent implant procedures at 19 centres between September 2019 and November 2020 (Supplementary Table 1). The mean age was 83.5±5.4 years, 58.3% were female, the mean STS score was 4.0±2.0%, with 83.3% having at least one frailty factor and 18.3% deemed at extreme surgical risk. The majority (56.7%) of subjects were in NYHA Functional Class III or IV (Table 1).
Table 1. Baseline characteristics.
| Baseline characteristics | N=120 | |
|---|---|---|
| Demographics | Age (years) | 83.5±5.4 |
| Female | 58.3% | |
| NYHA Functional Class | II | 43.3% |
| III | 55.0% | |
| IV | 1.7% | |
| STS-PROM scorea (%) | 4.0±2.0 | |
| <4% | 60.0% | |
| 4-8% | 35.8% | |
| >8% | 4.2% | |
| EuroSCORE II (%) | 3.6±2.5 | |
| Extreme risk | 18.3% | |
| Hypertension | 78.3% | |
| Diabetes mellitus | 27.5% | |
| Oral-controlled | 17.5% | |
| Kidney disease | 25.8% | |
| Atrial fibrillation | 26.7% | |
| Permanent pacemaker | 10.8% | |
| Pre-existing RBBB | 11.7% | |
| Prior transient ischaemic attack | 7.5% | |
| Carotid artery disease | 6.7% | |
| Coronary artery disease | 61.7% | |
| Prior coronary stenting | 24.2% | |
| Prior coronary artery bypass graft | 11.7% | |
| Prior myocardial infarction | 8.3% | |
| Peripheral vascular disease | 9.2% | |
| Chronic lung disease | 27.5% | |
| Hostile chest/prohibitive chest deformity | 1.7% | |
| Porcelain aorta | 0.0% | |
| Severe liver disease | 0.8% | |
| Pulmonary hypertension | 15.0% | |
| Non-ambulatory | 1.7% | |
| Frailty indices | Number of frailty indices | 1.4±0.9 |
| 0 | 16.7% | |
| 1 | 39.2% | |
| 2 | 36.7% | |
| 3 | 6.7% | |
| 4 | 0.8% | |
| Katz Index of Activities of Daily Living (≤4) | 3.3% | |
Grip strength (| 76.7% |
| |
| 15-foot walk test (≥height and sex-based cut-off) | 47.9% | |
| Albumin (<3.5 g/dl) | 11.7% | |
| Sizing and echocardiographic parameters | Annulus mean diameterb (mm) | 23.7±1.8 |
| Aortic valve area (cm2) | 0.7±0.2 | |
| Mean gradient (mmHg) | 46.7±13.4 | |
| Ejection fraction (%) | 60.3±8.7 | |
| Mitral insufficiency (moderate/severe) |
17.5% | |
| Tricuspid insufficiency (moderate/severe) | 12.5% | |
| Data are % (n/N) or mean±SD (n). aSubjects screened after 15 November 2018 were evaluated using risk models developed using STS data from 2011 to 2014 and validated using 2014 to 2016 data. bMeasurement from site computed tomography. BMI: body mass index; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association; RBBB: right bundle branch block; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | ||
Procedural characteristics and outcomes
Procedural characteristics are described in Table 2. Conscious sedation was used in one-third of subjects (33.3%). Most subjects (99.2%) were implanted via transfemoral access, with one subject undergoing TAVI via transaxillary access. The FlexNav DS integrated sheath was utilised in 84.2% of cases. The Navitor THV was resheathed at least once in 55 subjects (45.8%); the most common reason for resheathing was the initial valve placement being too high. Predilatation (recommended per the IFU) was performed in 92.5% of cases, and post-implant dilatation was performed in 32.5% of subjects. The 27 mm Navitor THV was the most implanted valve size (35.0%), followed by the 25 mm (30.8%) and 29 mm (30.8%) valve sizes; the 23 mm valve was implanted least often (3.3% of cases). The mean implant depth, estimated by fluoroscopy (site-reported), was 3.5±1.9 mm from the non-coronary cusp and 4.8±2.0 mm from the left coronary cusp.
Procedural outcomes are presented in Table 3. Of the 120 subjects, 117 (97.5%) were implanted with a single Navitor THV and three subjects (2.5%) required a second valve to be implanted during the index procedure due to a supra-annular positioning of the first valve. All three subjects received a second Navitor THV. There were no procedural deaths or conversions to surgical aortic valve replacement.
Table 2. Procedural characteristics.
| Procedural characteristics | N=120 | |
|---|---|---|
| Conscious sedation anaesthesia | 33.3% | |
| Total procedure timea (min) | 72.8±25.0 | |
| Total implantation timeb (min) | 12.1±7.9 | |
| Total fluoroscopy time (min) | 20.7±8.7 | |
| Volume of contrast (cc) | 120.5±63.5 | |
| Use of FlexNav DS integrated sheath | 84.2% | |
| Pre-TAVI balloon valvuloplasty | 92.5% | |
| Access route | Transfemoral | 99.2% |
| Subclavian/axillary | 0.8% | |
| Any resheathing performed | 45.8% | |
| 1 resheath | 31.7% | |
| 2 resheaths | 8.3% | |
| >2 resheaths | 14.2% | |
| Navitor valve size implanted | 23 mm | 3.3% |
| 25 mm | 30.8% | |
| 27 mm | 35.0% | |
| 29 mm | 30.8% | |
| Post-TAVI balloon valvuloplasty | 32.5% | |
| Implant depth (mm) | 4.2±1.8 | |
| Non-coronary sinus (mm) | 3.5±1.9 | |
| Left coronary sinus (mm) | 4.8±2.0 | |
| Length of hospital stay (days) | 3.0±2.4 | |
| Data are % (n/N) or mean±SD (n). aTotal procedure time: procedure start time is defined as vascular access time; procedure end time is defined as the time of removal of the last delivery system. bTotal implant time: implant start time is defined as the time of introduction of the delivery system from first attempted valve inserted into the body; implant end time is defined as the time of last attempted valve being fully deployed. DS: delivery system; SD: standard deviation; TAVI: transcatheter aortic valve implantation | ||
Table 3. Procedural outcomes.
| Procedural outcomes | N=120 |
|---|---|
| Procedural successa | 97.5% |
| Procedural mortality | 0.0% |
| TAVI-in-TAVI | 2.5% |
| Conversion to surgical aortic valve replacement | 0.0% |
| No TAVI valve implanted | 0.0% |
| aDefined as absence of procedural mortality and correct positioning of a single prosthetic heart valve into the proper anatomical location. TAVI: transcatheter aortic valve implantation | |
Clinical outcomes
Clinical outcomes at both 30 days and 1 year are presented in Table 4. At 30 days, the primary endpoint of all-cause mortality was 0.0%. Disabling stroke and major vascular complications occurred in 0.8% of subjects, life-threatening bleeding occurred in 2.5% of subjects, and no subjects experienced stage 3 AKI. The one major vascular complication was associated with the non-TAVI vascular access site. The non-hierarchical composite clinical endpoint was 4.2%.
Permanent pacemaker implantation (PPI) within 30 days occurred in 16 subjects (13.3% of all 120 subjects and 15.3% of pacemaker-naïve subjects). Of the 16 subjects who required new PPI, 13 had pre-existing conduction abnormalities (e.g., atrial fibrillation, first degree atrioventricular block, right bundle branch block). Subjects with new PPI at 30 days had a deeper implant (on average) compared to those without new PPI, based on both the mean stent protrusion into the left ventricular outflow tract (4.8 mm vs 4.2 mm) and depth from the side of the non-coronary cusp (4.4 mm vs 3.5 mm).
At 1 year, all-cause mortality was 4.2%, and life-threatening bleeding occurred in 5.0% of subjects. No subjects experienced additional disabling stroke, stage 3 AKI, or major vascular complication events. The non-hierarchical composite clinical endpoint was 9.2% at 1 year. Permanent pacemaker implantation up to 1 year occurred in 18 subjects (15.0% of all 120 subjects and 16.8% of pacemaker-naïve subjects). Other descriptive clinical outcomes at 30 days and 1 year are shown in Table 4.
Table 4. Clinical outcomes at 30 days and 1 year.
| Clinical outcomes | 30 daysa % (n) | 1 yearb % (n) | |
|---|---|---|---|
| Composite safety endpointc | 4.2% (5) | 9.2% (11) | |
| All-cause mortality | 0% (0) | 4.2% (5) | |
| Cardiovascular mortality | 0% (0) | 1.7% (2) | |
| Neurological events | Disabling stroke | 0.8% (1) | 0.8% (1) |
| Non-disabling stroke | 0.8% (1) | 2.5% (3) | |
| Transient ischaemic attack | 0.8% (1) | 2.5% (3) | |
| Bleeding | Life-threatening bleeding | 2.5% (3) | 5.0% (6) |
| Requiring transfusion | 1.7% (2) | 1.7% (2) | |
| Major bleeding | 2.5% (3) | 2.5% (3) | |
| Acute kidney injury | Stage 1 | 0.8% (1) | 0.8% (1) |
| Stage 2 | 1.7% (2) | 1.7% (2) | |
| Stage 3 | 0% (0) | 0% (0) | |
| Major vascular complications | 0.8% (1) | 0.8% (1) | |
| Ipsilateral access-site related | 0% (0) | 0% (0) | |
| Contralateral access-site related | 0.8% (1) | 0.8% (1) | |
| Overall permanent pacemaker (n=120) | 13.3% (16) | 15.0% (18) | |
| New permanent pacemaker (n=107) | 15.0% (16) | 16.8% (18) | |
| aBy proportion. bBy Kaplan-Meier estimation, 0-365 days post-index procedure. cNon-hierarchical composite of all-cause mortality, disabling stroke, life threatening bleeding, stage 3 acute kidney injury, or major vascular complications. | |||
Echocardiographic outcomes
Valve haemodynamics up to 1 year are presented in Figure 1. The mean transvalvular gradient decreased from 42.7±13.8 mmHg at baseline to 7.4±3.5 mmHg at 30 days and was sustained up to 1 year (7.5±3.2 mmHg). The mean EOA increased from 0.7±0.2 cm2 at baseline to 2.0±0.5 cm2 at 30 days and was sustained up to 1 year (1.9±0.4 cm2). Paired analysis demonstrated a significant improvement in the mean transvalvular gradient (43.5±14.0 mmHg to 7.5±3.2 mmHg, n=107) and EOA (0.7±0.2 cm2 to 1.9±0.2 cm2, n=84) between baseline and 1 year (p<0.0001 for both).
PVL at 30 days was none or trace in 79.7% and mild in 20.3% of subjects; no subjects had moderate or severe PVL (Central illustration). At 1 year, PVL was none or trace in 72.1%, mild in 26.9%, and moderate in 1.0% of subjects; no subjects had severe PVL at 1 year.
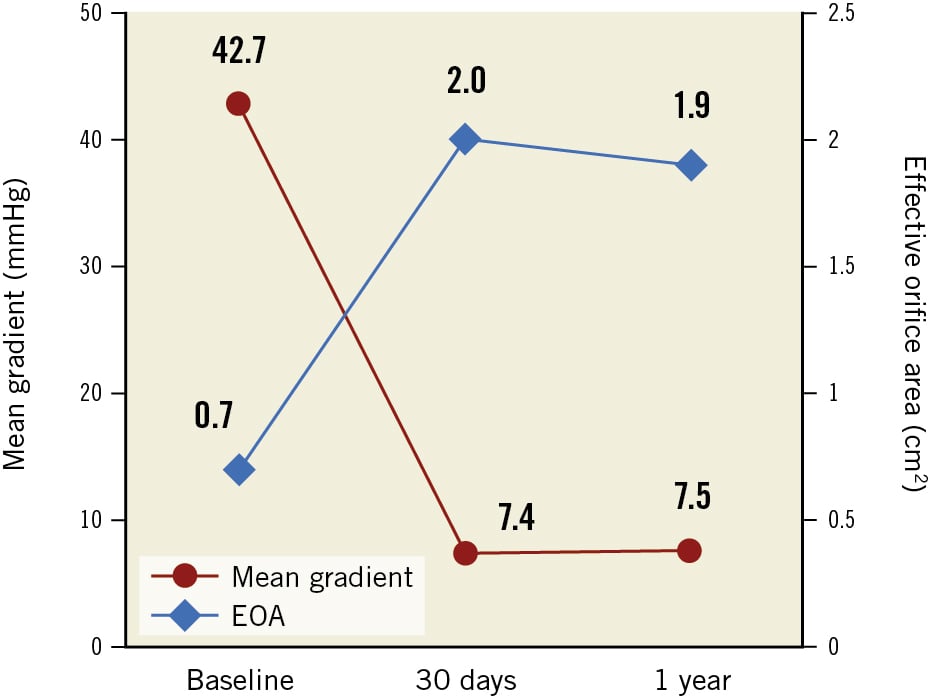
Figure 1. Aortic valve haemodynamics as assessed by an independent core lab. Effective orifice area (EOA) and mean transvalvular gradient at baseline, 30 days, and 1 year. Large EOAs and single-digit mean transvalvular gradients are observed at 30 days and sustained up to 1 year
Functional and quality-of-life status
An improvement in NYHA Class was observed following implantation of the Navitor THV (Figure 2). Subjects exhibited a marked improvement in NYHA Class at 30 days, which was maintained at 1 year. Most subjects were classified as NYHA Class I or II at both 30 days (96.6%) and 1 year (97.2%), with no subjects in NYHA Class IV. Most subjects (85.2%) improved by at least 1 NYHA Class from baseline to 1 year in a paired analysis (Supplementary Table 2).
Two quality-of-life measurements show significant improvement at 30 days compared to baseline (Figure 3). Scores from the SF-36 quality-of-life questionnaire improved in paired subjects from baseline to 30 days for both the physical (+4.89 points; p<0.0001) and mental (+2.50 points; p<0.02) components. Additionally, the distance walked in the six-minute walk test increased by 21.0 metres (p<0.01) in paired subjects from baseline to 30 days.
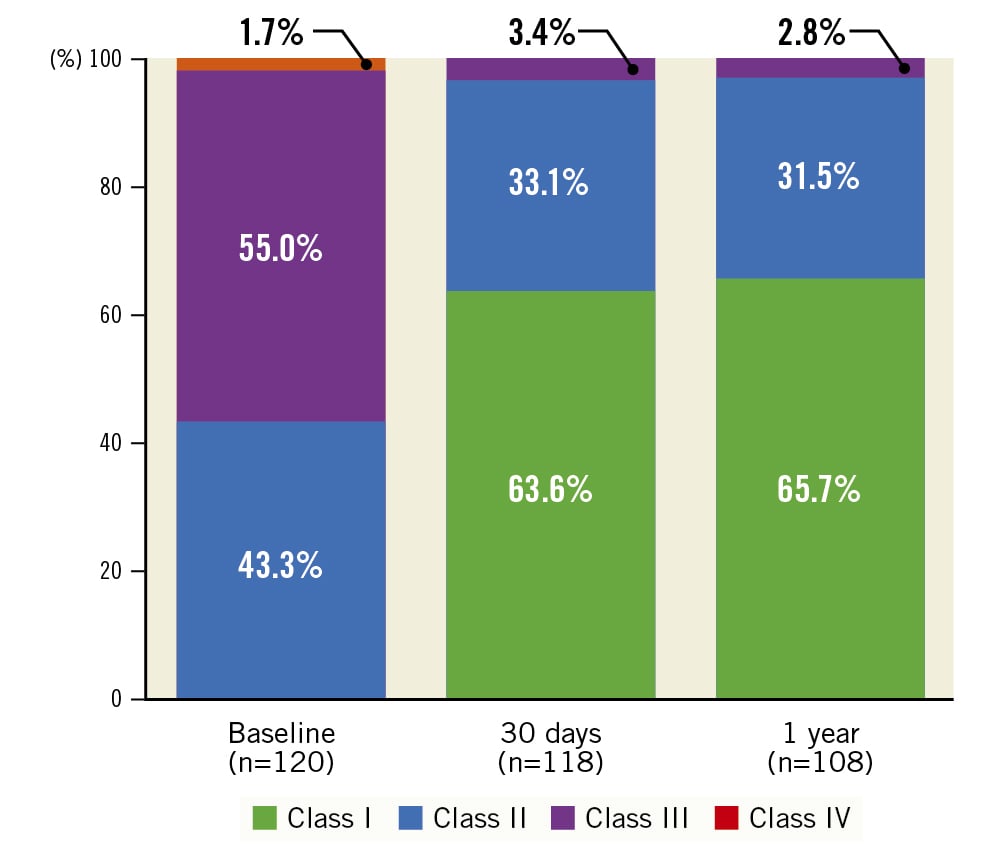
Figure 2. New York Heart Association (NYHA) Functional Class. Results demonstrate a large improvement from baseline to 30 days, and this improvement is sustained at 1 year with a majority of subjects in NYHA Class I or II
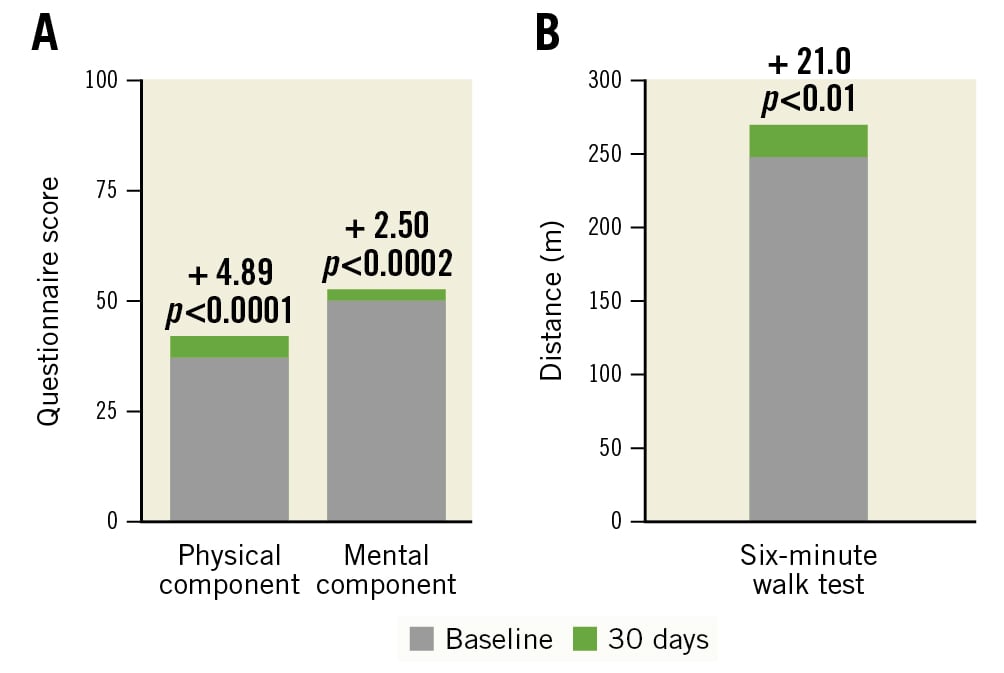
Figure 3. Quality-of-life measurements and 6-minute walk test (paired subject analysis). A) The SF-36 questionnaire responses indicate a significant increase in quality of life from baseline to 30 days in paired subjects (N=118) for both the physical and mental components. B) Subject distance increased significantly, by 21.0 m from baseline to 30 days, in the six-minute walk test for paired subjects (N=94)
Discussion
The main findings of this prospective, multicentre, global, single-arm, investigational study are as follows: 1) a high rate of procedural success, 2) low rates of mortality at 30 days and 1 year, and 3) low rates of PVL at 30 days and 1 year.
Procedural success was high at 97.5%. All subjects received a Navitor THV; three subjects required an additional Navitor THV due to an initial high deployment. No subjects required conversion to SAVR, and there was no procedural mortality. Only one (0.8%) major vascular complication occurred, which was at the contralateral access site. The major vascular complication rate is consistent with other contemporary THV systems, such as the SAPIEN 3 Ultra (1.1%; Edwards Lifesciences), Evolut PRO (10.0%; Medtronic), and ACURATE neo2 (3.3%; Boston Scientific) transcatheter heart valves8910.
Overall, acute safety outcomes in the CE mark cohort are favourable and are within expected ranges for a high or extreme surgical risk population. The non-hierarchical composite safety endpoint was 4.2%, which is lower than that which has been reported for both the Evolut PRO (15.0%) and the ACURATE neo2 (13.3%) transcatheter heart valves910. It is important to note that the composite event rates for both the Evolut PRO and ACURATE neo2 included all stroke, stage 2/3 AKI, coronary artery obstruction and valve-related dysfunction requiring repeat procedure, in addition to the events reported in our composite safety endpoint. However, no coronary obstruction or valve-related dysfunction was reported at 30 days for the Evolut PRO, and the only reported stroke was a disabling stroke, thus, this composite is effectively the same as our endpoint. The ACURATE neo2 composite endpoint included one stroke, graded as less than disabling, and one coronary obstruction.
An all-cause mortality rate of 0% at 30 days in this cohort compares favourably with other THVs (1.7%-3.3%) studied in a similar patient population91011. Event rates of the other components of the safety composite endpoint in this cohort are within the range or numerically lower for published outcomes of the SAPIEN 3, Evolut PRO, and ACURATE neo2 valves, including disabling stroke (0.8% vs 0.9%-1.7%), life-threatening bleeding (2.5% vs 5.0%-11.7%), AKI stage 2/3 (1.7% vs 0.8%-1.7%), and major vascular complication (0.8% vs 3.3%-10.0%), for a high or extreme surgical risk population91011. These outcomes suggest the Navitor THV performs similarly to other contemporary THVs.
Furthermore, safety outcomes at 1 year were favourable in subjects who received a Navitor THV compared to its contemporaries. The rates of all-cause mortality (4.2%) and disabling stroke (0.8%) in this study compare favourably to other contemporary THVs (11.8%-14.4% for all-cause mortality and 1.7%-2.4% for disabling stroke)91012.
New PPI remains a common concern following TAVI, as this has been associated with worse clinical outcomes (i.e., higher mortality, increased hospitalisation for heart failure) and increased cost131415. The rate of new PPI was 15.0% at 30 days in the CE mark cohort. This rate is similar to the reported PPI rates for the Evolut PRO (11.8%), ACURATE neo2 (15.0%), and SAPIEN 3 (13.3%) THVs in prospective studies with a similar high- or extreme-risk population91011. Of the 16 subjects who required a new PPI in our study, 13 had pre-existing conduction abnormalities. In addition, procedural factors like implant depth and procedural manipulations (e.g., resheathing) that interfere with the conduction system can result in the need for a permanent pacemaker.
Valve haemodynamics were favourable at 30 days, with single-digit mean gradients and large EOA, and this was sustained up to 1 year. These results demonstrate a similar performance to self-expanding valves with a supra-annular leaflet position and a numerically better performance as compared to balloon-expandable valves8101216. One explanation for this finding is that the Navitor THV design contains a cylindrical inflow portion of the stent frame allowing for better opening of the leaflets. These results align with a previous randomised control study comparing the Portico THV platform to both types of commercially available valves17. In addition to haemodynamic performance, a major benefit of an intra-annular leaflet design over the supra-annular leaflet design, in combination with large stent cells, is preserved flow and access to the coronary arteries after deployment.
Design iterations of contemporary THVs include features to mitigate PVL. For example, the balloon-expandable SAPIEN 3 Ultra device includes an enhanced outer sealing skirt made from textured polyethylene terephthalate and is ~40% higher compared to its predecessor device8. The self-expanding Evolut PRO and ACURATE neo2 devices include modifications to reduce PVL compared to their predecessor devices; Evolut PRO includes an external pericardial wrap, while the ACURATE neo2 device includes a pericardial sealing skirt on the outer and inner surface of the stent frame1018. Unlike these contemporary THVs, the outer cuff of the Navitor THV (i.e., the NaviSeal cuff) actively synchronises to the cardiac cycle, seals, and works to mitigate PVL by expanding to fill calcification-related gaps between the annulus and the valve. The addition of the NaviSeal cuff has demonstrated a reduction in PVL at both 30 days and 1 year compared to its predecessor device. With more than 70% of patients experiencing none or trace PVL up to 1 year, the NaviSeal cuff is functioning as intended. The rate of moderate PVL at 1 year in this study (1.0%) is within the range of both self-expanding (0%-2.7%) and balloon-expandable (0.6%-2.7%) contemporary TAVI devices with features to reduce PVL8101216.
Conclusions
Patients implanted with the Navitor THV have demonstrated favourable clinical outcomes, as is evident from the low rates of VARC-2 events, improved symptoms and quality of life, single-digit mean gradients, large EOAs, and low rates of PVL up to 1 year. These results demonstrate the safety and effectiveness of the Navitor THV in treating high or extreme surgical risk patients with severe symptomatic aortic stenosis.
Impact on daily practice
The Navitor THV, with an intra-annular leaflet position, offers favourable clinical outcomes and haemodynamics up to 1 year. The active outer NaviSeal cuff demonstrates a reduction in PVL.
Acknowledgments
The authors would like to thank all the investigators and institutions participating in the PORTICO NG Study. They thank Kai Koo, PhD and Feiyi Jia, PhD (Abbott) for their contributions to data analysis.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg − Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
L. Sondergaard has received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and Sahajanand Medical Technologies Ltd. A.S. Walton is a proctor for Abbott, Medtronic, and Edwards Lifesciences; serves on medical advisory boards for Abbott, Medtronic, and Edwards Lifesciences; and receives grant support from Abbott, Medtronic, and Edwards Lifesciences. S.G. Worthley reports speaker fees for Abbott, Edwards Lifesciences, and HighLife Medical; is a consultant for Abbott, Edwards Lifesciences, HighLife Medical, and Procyrion; has a proctorship for Abbott, Edwards Lifesciences, and HighLife Medical; and is a shareholder in Three Peaks Medical. D. Smith reports speaker and proctor fees from Abbott. B. Chehab reports speaker, consultancy and proctor fees from Abbott, Edwards Lifesciences, Medtronic, and CSI. G. Manoharan is a proctor for Abbott and Medtronic. G. Yong is a proctor for Abbott. F. Bedogni reports proctor, speaker, and consultant fees from Abbott, Medtronic, Boston Scientific, and Meril. N. Bates is an employee of Abbott. M.J. Reardon reports consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and Gore Medical. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

