Abstract
Aims: Transcatheter aortic valve implantation has emerged as an alternative to conventional aortic valve replacement in high-risk patients. Diverse prostheses are currently under investigation. The aim of this study was the clinical, safety and efficacy assessment of Braile Inovare Transcatheter Aortic Prosthesis usage.
Methods and results: Ninety high-risk or inoperable patients underwent transcatheter aortic valve implantation. The mean logistic EuroSCORE was 39.3%. All patients presented calcified aortic stenosis. The procedures were performed under fluoroscopic and echocardiographic guidance. Prostheses were implanted through the transapical approach under rapid ventricular pacing. Echocardiographic and angiographic controls were included. Implantation was feasible in 87 cases. There was only one case of operative mortality, and 30-day mortality was 13.3%. The median transvalvular aortic gradient was reduced from 44.8±15.3 to 14.1±8.0 mmHg. Left ventricular function improved in the first seven postoperative days. Paravalvular aortic regurgitation was present in 29.7% of cases, mostly trace. One case presented a major vascular complication, and there were two cases of permanent pacemaker implantation. Two cases of major stroke occurred.
Conclusions: Transcatheter aortic valve replacement using the Braile Inovare prosthesis is able to provide encouraging results with significant functional and structural cardiac improvement. It is mandatory to continue follow-up to measure the benefits of this device as well as to improve selection criteria of patients.
Introduction
Calcified aortic stenosis is considered one of the most frequent causes of aortic stenosis in developed countries. After the onset of symptoms, the prognosis is very poor1. Standard treatment consists of aortic substitution with relatively low mortality2. In addition to these results, some patients present very high surgical risk due to multiple comorbidities, thus justifying procedure contraindication. In this context, transcatheter aortic valve replacement (TAVR) has emerged as an alternative for this high-risk patient subset3.
Multiple studies have demonstrated the feasibility and safety of TAVR. Recent clinical trials have also demonstrated comparable results to standard surgical aortic valve replacement (SAVR) in selected cases and superiority compared with the clinical alternatives4.
Diverse prosthesis types are now under investigation in multiple trials with various advantages and disadvantages. In addition, major differences in selection criteria have led to significant discrepancies between different study populations, making comparisons difficult5,6.
The objective of this study is to report the clinical results of up to four years of follow-up with the new Braile Inovare prosthesis (Braile Biomedical, São José do Rio Preto, Brazil).
Methods
PATIENT SELECTION AND INCLUSION CRITERIA
Between June 2008 and July 2013, 90 patients underwent transcatheter aortic valve implantation after institutional ethical review board approval (registration number 1116/08) at a single centre. All patients provided informed consent. Information regarding the initial patients of this series has already been published5.
A multidisciplinary team was responsible for the selection of patients with severe aortic stenosis and at high risk for conventional surgery. Selection also included aspects such as frailty, surgeon perception, current quality of life and life expectancy. Frailty was judged by multiple factor analysis including body mass index, albumin, weight loss, hand grip strength, gait speed, five-minute walk test and Katz activity score. EuroSCORE and STS score were also included in an attempt to provide a quantitative mortality and morbidity assessment. Complete selection criteria have been published previously7.
Subjects underwent laboratory, echocardiogram, coronarography, iliac-femoral Doppler ultrasound and carotid-vertebral Doppler ultrasound evaluation. The inclusion and exclusion criteria have been previously published but included the presence of severe aortic stenosis and a very high or unacceptable surgical risk. An anatomical inclusion criterion also had to be met in that the calcified annulus had to be in the 17 mm to 28 mm range. Patient characteristics are listed in Table 1.
DEVICE AND PROCEDURE
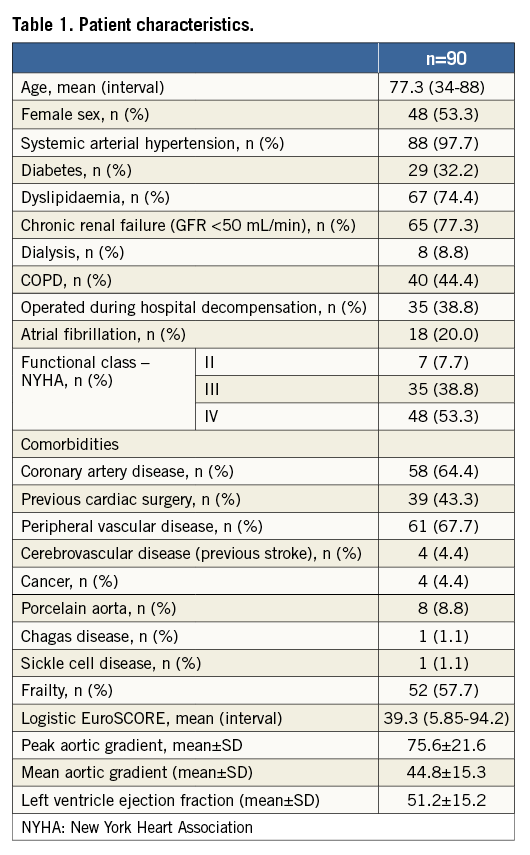
The Braile Inovare prosthesis was used in all cases. The valve is a balloon-expandable prosthesis with a lozenge cobalt-chromium frame, 20 mm height, three radiopaque markers (identifying base, valve and skirt) and a single sheet of bovine pericardium composing the leaflets in the following diameters: 20, 22, 24, 26, and 28 mm (Figure 1). The prosthesis is already commercially available in Brazil. It differs in several respects from other available valves, especially the Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA). The main different features include a wider range of diameters (better size selection avoiding excessive over/undersizing), the presence of more vertices in the lozenge frame (for better force distribution and annulus accommodation, possibly contributing to decreased conduction disturbances), usage of a single pericardial sheet for all leaflets (reducing the number of pericardial sutures), and increased height.

Figure 1. Braile Inovare aortic prosthesis.
The delivery system is composed of a 24 Fr transapical introducer sheath for all valve sizes. The implantation process is summarised as follows: after anaesthetic induction, the patient is placed in a supine position. A cardiopulmonary bypass system is kept on standby. Heparin at a dose of 2 mg/kg is administered in order to achieve an activated clotting time (ACT) above 350 seconds (in interventional procedures using the transapical approach we use a 2 mg/kg heparin dose/ATC >350 s).
A femoral artery is punctured using the Seldinger technique, and a 6 Fr introducer is positioned. A catheter is advanced into the aortic root with the aid of a guidewire, in order to allow the performance of aortography before and after prosthetic valve opening, as well as identification of the aortic sinuses and coronary ostia. The ventricular apex is identified by transthoracic echocardiography. At the marked site, an incision of approximately 5 cm in length is made in order to obtain access to the ventricular apex. The ventricular apex is then punctured with a 6 Fr introducer. A stiff guidewire is positioned across the stenotic valve, the 6 Fr introducer is withdrawn, and a 24 Fr introducer is placed into position.
A balloon catheter of appropriate diameter is placed on the aortic valve. Then, a controlled hypotension is carried out with the aid of rapid ventricular pacing. Thereafter, the balloon is inflated to its maximum nominal pressure in order to promote aortic valvuloplasty. The balloon is then deflated. The pressure is now restored and a control echocardiogram is performed to confirm the effectiveness of the valvuloplasty. The balloon is then removed and replaced over the same guidewire by the previously mounted valve prosthesis. A new episode of controlled hypotension is carried out and then the balloon catheter with the prosthesis mounted is inflated to its maximum nominal pressure in order to promote the release of the prosthesis. The introducer is removed and the ventricular apex closed using a purse-string suture. After apical closure, we use protamine in a 1:1 dose. Protamine is administered using a peripheral venous line in a slow infusion (five to ten min).
All implants were performed transapically in a hybrid operation room with the previously described technique, using fluoroscopic and transoesophageal echocardiographic images. In addition, 10% oversizing was used to select adequate prosthesis diameter based on intraoperative transoesophageal echocardiography and computed tomography (Figure 2).
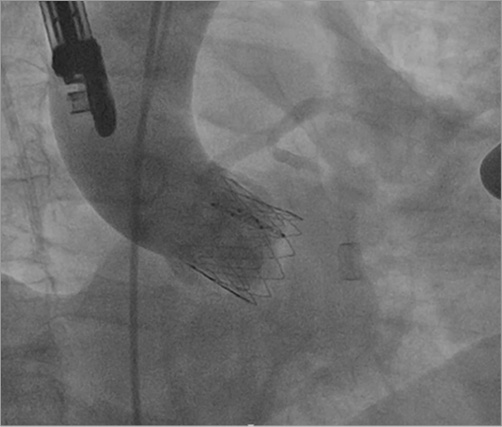
Figure 2. Inovare prosthesis implanted in aortic annulus Aortography showing no residual aortic insufficiency and no coronary occlusion.
Immediately after prosthesis deployment and haemodynamic stabilisation, transoesophageal echocardiography was performed to assess prosthesis function, transvalvular gradient and regurgitation. In the presence of perivalvular aortic regurgitation, another ballooning episode was performed under rapid ventricular pacing. Aortography was performed only if any coronary obstruction or prosthesis malfunctioning were suspected, in order to avoid excessive contrast dye utilisation.
After the procedure, all patients were administered double antiplatelet therapy (ASA+clopidogrel) for six months, and then ASA only unless contraindicated for clinical reasons.
FOLLOW-UP AND OUTCOMES
Procedural success was defined as a correct implantation, satisfactory haemodynamic profile, no significant valvular or perivalvular regurgitation, no interference with the coronary ostia and absence of major complications, including conversion to open surgery. Patients were followed prospectively (postoperative day one, seven, 30, 180, 360, 540, 720 and then annually) with clinical and echocardiographic evaluation.
The primary endpoint was the rate of death from any cause. The following outcomes were analysed: all-cause mortality, cardiovascular mortality, functional class (NYHA), echocardiography evolution and complications. The outcomes analysis followed the Valve Academic Research Consortium recommendations8.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS version 20 software (IBM Corp., Armonk, NY, USA). The significance level was 0.05. Mean comparisons (echocardiographic data) were evaluated using the Friedman test after a normal distribution test. Continuous variables are reported as means ±SD unless stated differently. Kaplan-Meier estimates were used to construct the survival curves on the basis of all available follow-up data for the time-to-event analyses.
Results
PROCEDURE
All cases were performed in a hybrid operating room. Successful implantation was feasible in 87 cases. There were three immediate conversions to open surgery, two due to prosthesis migration and one due to haemodynamic instability after deployment.
The mean procedure time was 136.2±81.8 minutes. The mean fluoroscopic time was 11.5±4.9 minutes. Mean contrast dye use was 29.0±30.1 mL. Operative mortality was observed in one case. Only one valve was used in all patients (20 mm in 3.3%; 22 mm in 14.4%, 24 mm in 40%, 26 mm in 36.6%, and 28 mm in 5.5%). A major vascular complication occurred in one case (iliac vein rupture during femoral cannulation) without mortality (used due to haemodynamic instability). Permanent pacemaker implantation was necessary in two cases, one slow ventricular response atrial fibrillation with haemodynamic compromise (15th postoperative day) and one complete atrioventricular block after discharge (45th postoperative day), for a total of 2.2%.
Follow-up varied from one to 48 months (median 481.6 days). Only one case was lost to follow-up. The operative variables are listed in Table 2. All patients were followed by the Transcatheter Heart Valve Group in the outpatient clinic.
MORTALITY AND READMISSION
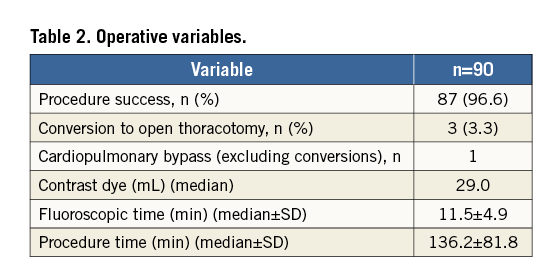
Thirty-day mortality was 13.3%. Survival at six, 12, 18, 24, 36 and 48 months according to the Kaplan-Meier analysis was 65.1%, 62.7%, 61.3%, 58.3%, 54.3% and 53.3%, respectively. Survival after discharge according to Kaplan-Meier analysis at six, 12, 18, 24, 36 and 48 months was 87%, 85%, 83%, 83%, 77.5% and 77.5%, respectively (Figure 3). Mortality causes at 30 days (12 patients) included sepsis with bloodstream inflection (six cases), stroke (one case), bronchopneumonia (five cases).
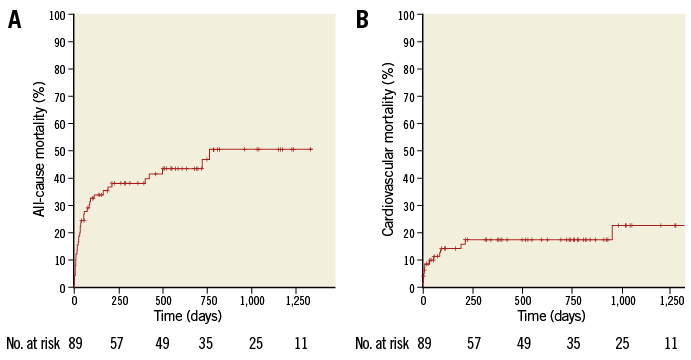
Figure 3. Kaplan-Meier mortality curves. A) All-cause mortality. B) Cardiovascular mortality.
The readmission rate was 8.8%. After the sixth month, we had only one readmission due to endocarditis requiring conventional surgery and prosthesis removal without death. Readmission causes included pneumonia (H1N1), haemothorax, left pleural effusion, aortic insufficiency (new ballooning episode), cardiogenic shock, AV block, leukaemia and cardiac insufficiency.
Complications
We analysed the diverse complications according to the VARC criteria. Major stroke occurred in two patients, and no minor or transient ischaemic attack was noted (no neuroimaging modalities were used in patients without clinical stroke signs in order to document silent ischaemic events). Major vascular complications occurred in one patient (iliac vein rupture during cannulation for haemodynamic stabilisation with cardiopulmonary bypass). Minor vascular complications occurred in six patients who required additional blood transfusion due to difficult apical closure. Diverse complications are described in Table 3.
FUNCTIONAL CLASS
Functional class (NYHA) significantly improved over time when compared to preoperative level. Comparison between one, six, 12, 24 and 36 months revealed a sustained improvement in functional class.
ECHOCARDIOGRAPHY EVALUATION
The haemodynamic results were evaluated by seriate echocardiography. A significant and sustained reduction in peak and mean gradient was achieved (peak 75.6±21.6 to 27.4±14.6 mmHg and mean 44.8±15.3 to 14.1±8.0 mmHg) at the first postoperative evaluation (p<0.001) (Figure 4).
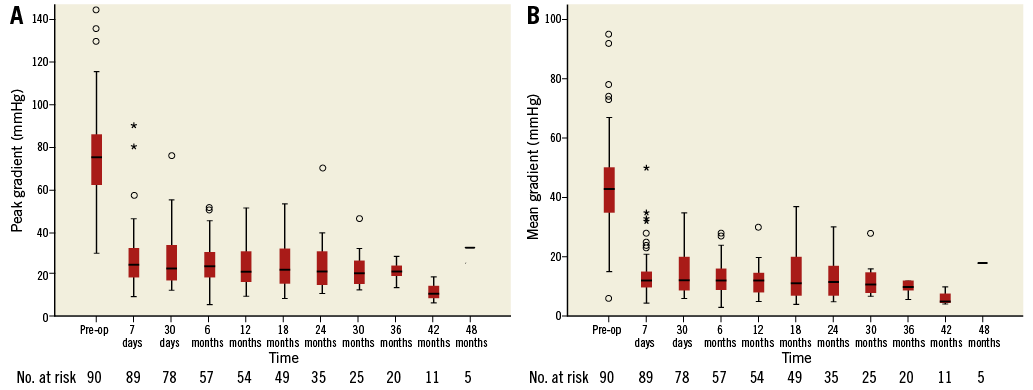
Figure 4. Aortic gradients. A) Peak aortic gradient. Pre-operation versus first post-operation evaluation – p<0.001. First post-operation versus subsequent operation evaluation – p>0.05. B) Mean aortic gradient. Pre-operation versus first post-operation evaluation – p<0.001. First post-operation versus subsequent operation evaluation – p>0.05.
Postoperative aortic insufficiency was present in 30.0% of cases (none, 70%; trace, 23.3%; mild, 6.6%; moderate, 0%; and severe, 0%). No further aortic insufficiency was noted except in one case requiring a new ballooning episode. Left ventricular function, as evaluated by the Simpson method, also improved from 51.2%±15.2% to 57.1%±12.0% at the seventh postoperative day with sustained results (p<0.01) (Figure 5).
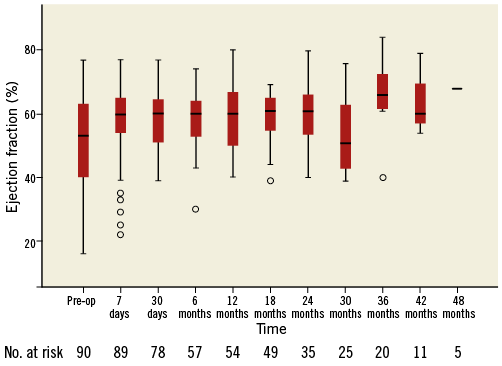
Figure 5. Left ventricular ejection fraction pre-operation, 7th day, one, six, 12, 18, 24, 30, 36 and 42 postoperative months. Pre-operation versus day 7 post-operation, p>0.05. Pre-operation versus one month, p=0.01. Pre-operation versus six months, p<0.0001. Pre-operation versus 12 months, p<0.0001. Pre-operation versus 18 months, p<0.0001. Pre-operation versus 24 months, p<0.0001. Pre-operation versus 30 months, p<0.0001. Pre-operation versus 36 months, p<0.0001 Pre-operation versus 42 months, p<0.0001.
Discussion
Conventional aortic valve replacement is the standard procedure for aortic stenosis and aortic bioprosthesis dysfunction. In most patients, surgical intervention is a low-risk procedure, promotes functional class benefit and also survival improvement compared to clinical treatment2. Even older patients can achieve acceptable results at large-volume centres9. In addition to this evidence, a significant number of patients do not undergo surgery for many reasons, including unacceptable risk, poor technical conditions precluding cardiopulmonary bypass and aortic cross clamp (porcelain aorta, chest irradiation, previous coronary artery bypass with patent grafts or multiple comorbidities)10. In this scenario, a less invasive alternative involving transcatheter aortic valve implantation, using an apical or femoral approach, has emerged as a possibility in selected patients. Multiple centres have published encouraging results, but mortality and complications are still an issue blocking widespread use in the lower-risk population3.
The Inovare device was developed in a partnership involving industry, university and government funding agencies. Previous reports have demonstrated its use in diverse positions, but, until now, no follow-up has been published7. In the initial phase of this new device, the selected population was composed of very high or unacceptable surgical risk patients, with the logistic EuroSCORE reaching a mean of 40.5%. This includes averages above most published studies11. This extended series includes patients still with a very high-risk profile but now more comparable to other series.
Transapical access was selected because it allows larger introducer sheaths and has fewer peripheral vascular complications. In addition, valve positioning is easier as is aortic crossing, favouring the development of the valve and lessening the learning curve. Several studies have reported increased mortality with the transapical approach, with different explanations including thoracotomy and apical bleeding complications. However, in most cases, transapical access is reserved for patients with no peripheral vascular access, which inherently increases surgical risk by precluding a direct comparison between both access routes12.
We acknowledge that femoral access could be the route of choice in multiple situations, and some complications reported in our study could be related to the apical route. We are currently testing a femoral device and, in the near future, comparisons of both alternatives will be possible, providing patients with an adequate solution based on individual anatomy selection.
Hospital mortality differs greatly among published studies and registries. Unfortunately, patient selection is also variable, and direct comparisons are difficult to achieve. In this study, mortality is under the predicted risk but improvements are still required in terms of patient selection and the learning curve. We also have to consider that 30-day mortality may not reflect the true patient outcome because patients could survive the initial procedure and die from multiple complications, such as infections (which are particularly dangerous in this frail group), after 30 days. The mortality levels achieved in this study were similar to those of other studies of extreme high-risk patients13,14.
Comparing our mortality to the PARTNER trial, we note similar follow-up results except for an initially worse mortality. In addition, there is a higher risk score in our population, which is most likely reflected in the 30-day mortality.
Minor complications are relatively frequent with a negative impact on follow-up, particularly in relation to vascular complications12. In our sample, only one major vascular complication occurred, but the apical access and relatively small sample size do not permit mortality impact analysis. Three patients required intermittent dialysis, even with 77.3% presenting a glomerular filtration rate of less than 50 mL/min. Most likely, the low use of contrast dye favourably contributed to the preservation of renal function.
Currently, it is difficult to determine the presence of structural valve deterioration because the follow-up time is relatively short. The longest follow-up achieved was 48 months, with no valvular deterioration measured by increasing gradients or new valve insufficiency. One patient required an episode of re-ballooning with a satisfactory final result, but the precise mechanism underlying this failure is not completely understood. Structure and leaflet compression during valve preparation could theoretically cause leaflet damage15. Diverse studies have reported the presence of leaflet damage after crimping and ballooning, but this is highly dependent on leaflet origin (bovine/porcine), preparation and thickness. Unpublished results concerning compression and ballooning tests using the Inovare pericardial leaflet did not indicate any damage.
Complete atrioventricular blockage is a frequently described complication, affecting one third of the patients in some samples, particularly in those using a self-expandable prosthesis16. Balloon-expandable prostheses achieve lower AV block incidence, having similar results over diverse trials with a rate of approximately 4.5%3. In our sample, AV block incidence was low, most likely due to characteristics such as multiple valve sizes, which allows more precise sizing and thus avoids exaggerated undersizing or oversizing and a possibly greater risk of AV block.
Accurate echocardiography follow-up is mandatory after transcatheter aortic valve implantation. The comparison between diverse devices and conventional prostheses is highly important. The haemodynamic profile and durability of these new prostheses demand constant evaluation because major differences exist when they are compared to conventional devices. There are still no reliable long-term data regarding these valves. Some studies have reported superior profiles for the new prosthesis even when compared to stentless devices. These differences could explain the fast ventricular function recovery noted in this sample by contributing a larger effective valve area, and the absence of aortic cross clamp and cardioplegia for aortic substitution17,18.
In addition to the favourable haemodynamic profile and reduced transvalvular gradients, the presence of aortic insufficiency in diverse degrees, particularly perivalvular, negatively affects outcomes. The persistence of small perivalvular leaks appears not to affect the outcomes, but moderate to severe leaks have been proven to be an independent mortality risk factor19.
The prevalence of aortic regurgitation in this series is higher than expected for conventional aortic valve substitution. Prosthesis improvement is needed to reduce this complication20. Possible mechanisms include the inability to adapt the prosthesis perfectly to the irregular aortic valve annulus, even after balloon aortic valvuloplasty, and also valve recoil after ballooning.
PARTNER trial data reveal a 10.5% prevalence of aortic regurgitation with periprosthetic origin after one year (moderate to severe)3. In the present study, the presence of moderate or severe aortic regurgitation at one year was smaller. The availability of multiple valve sizes allowed a more precise valve selection, making possible a better adjustment in relation to the aortic annulus.
Post-implant transvalvular gradients were significantly reduced when compared with preoperative levels. Sustaining these results over a follow-up period is consistent with the favourable profile of this new device, which is similar to other commercially available devices18.
Left ventricular function significantly improved in a short period. On postoperative day seven, it was already possible to note improvement. The rapid increase in left ventricular function could partially explain the mortality benefit of transcatheter valves over conventional procedures. The avoidance of cardiopulmonary bypass, aortic cross clamp and cardioplegic arrest associated with a smaller afterload generated by transcatheter valves could be one of the possible explanations for this17,18. Functional class also improved and remained stable over time. After 24 months, almost all patients are in NYHA Class I or II. Obviously, functional class evaluation should not be restricted only to NYHA, and other life quality measures should be assessed to evaluate functional improvement more precisely.
Limitations
The present study has some limitations including sample size and follow-up time. The learning curve is also present, particularly in the first half of the population, including selection criteria and conduct of the procedure. Correctly identifying the best candidates for this technique with better results over time will only be possible with increased patient numbers and longer follow-up time. Randomisation including clinical treatment, different access routes and conventional surgery will be able to confirm these favourable results.
Conclusion
Transcatheter aortic valve replacement using the Braile Inovare prosthesis is capable of providing encouraging results with increased functional and structural cardiac improvement. Sustained results were achieved in a 48-month follow-up. Follow-up needs to be continued to measure the benefits of this device, as well as its complications, and also to improve selection criteria. However, conventional surgical intervention is still the gold standard procedure for low-risk patients, and this technique should be reserved for highly selected patients.
| Impact on daily practice Transcatheter aortic valve implantation is now widely accepted for aortic stenosis treatment in selected high-risk patients. Diverse prostheses have been used with unique characteristics. The Inovare prosthesis is demonstrated to be safe and effective with comparable results to major commercially available transcatheter valves. It also provides a wider range of diameters, allowing the operator a better prosthesis selection, possibly contributing to reduced paravalvular leak and annular rupture risk. The design also allows valve-in-valve implants in aortic and mitral positions, representing an interesting alternative. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

