
Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is an established therapy for patients with severe aortic stenosis (AS). The aim of this study was to evaluate the newer-generation Portico TAVI system in an all-comers population.
Methods and results: This single-centre study included 216 patients with severe AS (Society of Thoracic Surgeons [STS] score 4.3±3.0%). The Portico valve was implanted using the transfemoral (91.2%), transsubclavian (5.6%) and transcaval (3.2%) access. Device success was achieved in 94.4% of cases. At 30 days, mortality and stroke rates were 2.3% and 0.5%, respectively. Early safety was achieved in 91.7% of cases. More-than-mild paravalvular leak (PVL), as assessed by echocardiogram, was observed in 3.4% of the patients, with rates of 4.9% and 1.9% in the first and second half of the cohort, respectively. A permanent pacemaker was implanted in 15.8% of those without prior pacemaker, with a rate of 11.1% in the second half of the cohort. At one year, incidence rates for all-cause mortality and stroke were 12.3% and 2.3%, respectively. In the low-risk group (STS <4%; n=128), Kaplan-Meier estimates at 30 days and one year were 0% and 7.5% for all-cause mortality and 0.8% and 2.2% for stroke, respectively. Haemodynamic improvements persisted over time with a mean transvalvular gradient of 7.0±3.0 mmHg at one-year follow-up.
Conclusions: The Portico TAVI system was safe to implant and achieved a high device success rate. With learning curve effects, the device achieves lower rates of PVL and pacemaker implantation and provides adequate clinical and haemodynamic outcomes up to one year.
Abbreviations
ASA: acetylsalicylic acid
AS: aortic stenosis
AVA: aortic valve area
LV: left ventricular
LVOT: left ventricular outflow tract
MSCT: multislice computed tomography
NYHA: New York Heart Association
PVL: paravalvular leak
RV: right ventricular
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
TTE: transthoracic echocardiography
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) is currently the treatment of choice for patients with symptomatic, severe aortic valve stenosis (AS) who are considered to be at increased surgical risk by a multidisciplinary Heart Team1. Ongoing research aims at further evolution of TAVI and expansion of its indications. Typical complications from first-generation TAVI devices include vascular injuries, paravalvular leak (PVL) and the need for implantation of a permanent pacemaker (PM)1,2. Although patient selection and operator experience may have explained some of these results, the characteristics of the first-generation TAVI devices may have contributed to these outcomes. Importantly, first-generation devices are not repositionable, which may result in suboptimal implant position and associated complications. The newer-generation Portico™ valve system (St. Jude Medical [now Abbott], St. Paul, MN, USA) is fully resheathable and repositionable. The objective of this study is to report the procedural and clinical outcomes obtained with this valve in a Danish all-comers population, comprising a large group of “low-risk” patients as defined by the Society of Thoracic Surgeons (STS) risk score.
Methods
PATIENT SELECTION AND DATA COLLECTION
Between April 2014 and June 2017, 216 consecutive patients with symptomatic severe AS underwent TAVI with the Portico system in Rigshospitalet, Copenhagen, Denmark. The Portico system has been the most commonly used transcatheter heart valve (THV) in our centre in the last few years; the only absolute exclusion criteria for the Portico valve have been an annulus perimeter >85 mm (approx. 20% of all patients) and non-calcified pure aortic regurgitation (less than 2% of all patients). All patients had been discussed by a multidisciplinary Heart Team and found eligible for TAVI based on surgical risk estimation, frailty, anatomical characteristics (e.g., porcelain aorta), age (>80 years), previous cardiac surgery, etc. In accordance with the institution’s policy, all patients gave written informed consent for the procedure and the use of anonymous data for research. All baseline patient and procedural data were prospectively collected in the Copenhagen TAVI Registry. Follow-up data were retrospectively collected by use of the Danish National Patient Register (Landspatientregisteret). Definition of device success, procedural safety and clinical efficacy, as well as classification of adverse events was according to the Valve Academic Research Consortium (VARC)-2 criteria2.
DEVICE DESCRIPTION
The Portico valve system is a newer-generation aortic bioprosthesis for transcatheter implantation, consisting of a self-expanding nitinol framework containing three bovine pericardium valve leaflets in an intra-annular position and a sealing cuff of porcine pericardium (Figure 1). Available valve sizes include 23, 25, 27 and 29 mm, allowing treatment of aortic valves with an annulus perimeter ranging from 60 to 85 mm. The delivery system is flexible, facilitating the crossing of the aortic arch. The valve prosthesis can be resheathed and repositioned before final deployment. The leaflets function at partial deployment and rapid pacing during deployment is not required in most cases, allowing more gradual deployment and minimising haemodynamic instability. The Portico valve is designed with a subannular sealing cuff to reduce PVL and features large stent cells to allow access to the coronary ostia. The inflow part of the stent frame is cylindrical with low extension into the left ventricular outflow tract (LVOT), which may minimise conduction system injury and the concomitant need for a permanent PM. It functions in both round and elliptical valve configurations, adapting to native valve geometry to minimise PVL.
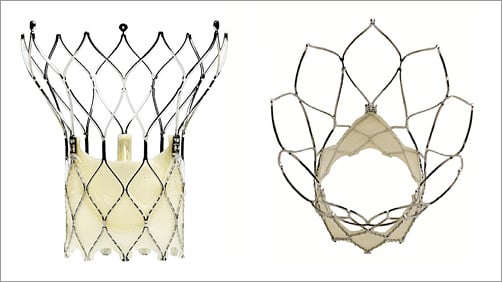
Figure 1. Portico™ aortic bioprosthesis (St. Jude Medical [now Abbott], St. Paul, MN, USA).
PROCEDURAL STEPS
All TAVI procedures were performed as previously described3. Transfemoral vascular access was the preferred access route; other access routes included the transsubclavian and transcaval approach. In most cases, the 18-19 Fr Ultimum™ sheath (St. Jude Medical [now Abbott]) was used to introduce the Portico valve; in 21 cases with small vessel diameter (4.5 to 5.0 mm), a sheathless approach was used as previously described4. Prosthetic valve size was selected based on multislice computed tomography (MSCT) measurements of the aortic valve annulus and its surrounding structures. Predilatation and/or post-dilatation was performed at the operator’s discretion. Predilatation was performed in most cases except for those cases with only mild leaflet calcifications; post-dilatation was considered in case of remaining PVL and/or THV underexpansion. All patients received a dose of 100 units/kg unfractionated heparin at the start of the TAVI procedure and a combination of clopidogrel (75 mg daily) and acetylsalicylic acid (ASA, 75 mg daily) for three months post procedure, followed by ASA lifelong. In case of indication for oral anticoagulant therapy, anticoagulation was combined with clopidogrel for three months; thereafter, anticoagulant therapy was continued lifelong.
STATISTICAL ANALYSIS
Descriptive statistics were expressed as mean±standard deviation (SD) for continuous variables (and median, interquartile range for STS score), and as frequency and percentage (%) for discrete variables. The differences in means between groups were determined using the Student’s t-test or Wilcoxon rank-sum test, whereas the chi-square test was used to test for associations between discrete variables. Statistical comparison was performed to compare early vs. later experience TAVI and to compare the mean gradient of the entire patient population before vs. after TAVI. A two tailed p-value <0.05 was considered to indicate statistical significance. Kaplan-Meier cumulative incidence curves were derived for mortality and stroke. Statistical analyses were performed using commercially available software, SPSS, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
STUDY POPULATION
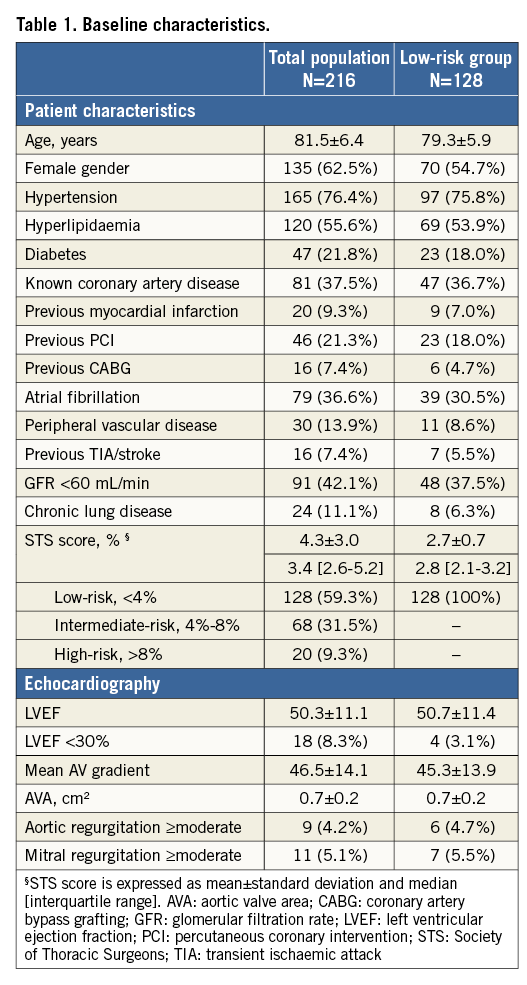
The study enrolled 216 patients with severe AS treated with a Portico valve: this represents 31% of all patients who underwent TAVI at our centre during this period. The mean age of the total study population was 82±6 years and 63% were female. The mean STS surgical risk score was 4.3%, with 128 patients (59%) being categorised as low surgical risk (STS score <4%). Table 1 summarises the baseline characteristics of the total and low-risk populations.
PROCEDURAL DATA AND OUTCOMES
A majority of TAVI cases were performed under local anaesthesia by the transfemoral approach. Baseline procedural data are shown in Table 2. Five cases involved TAVI in a previous surgical aortic bioprosthesis.
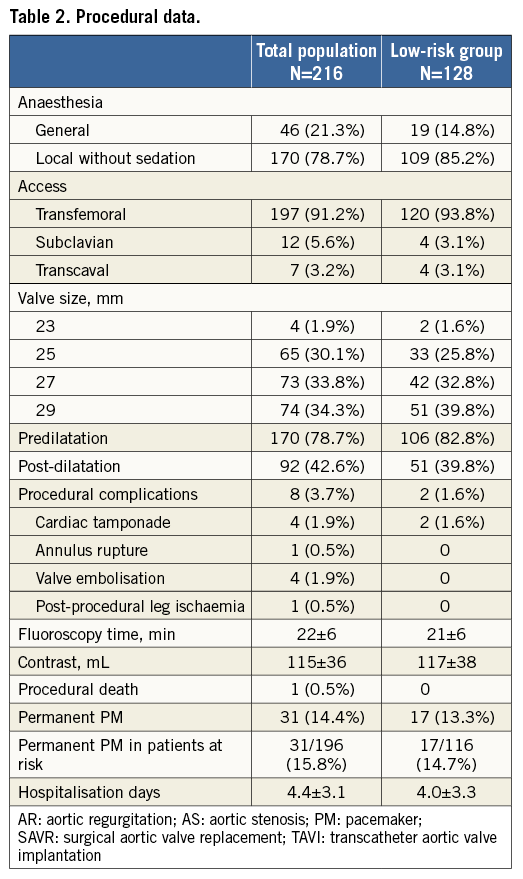
Device success was achieved in 94.4% of procedures. Failures to achieve device success were related to procedural death (n=1), device embolisation requiring a second valve (n=4), and more-than-mild PVL (n=8). Even better outcomes were observed in the low-risk group with a device success of 97.7% and a more-than-mild PVL rate of 2.3% (Table 3).
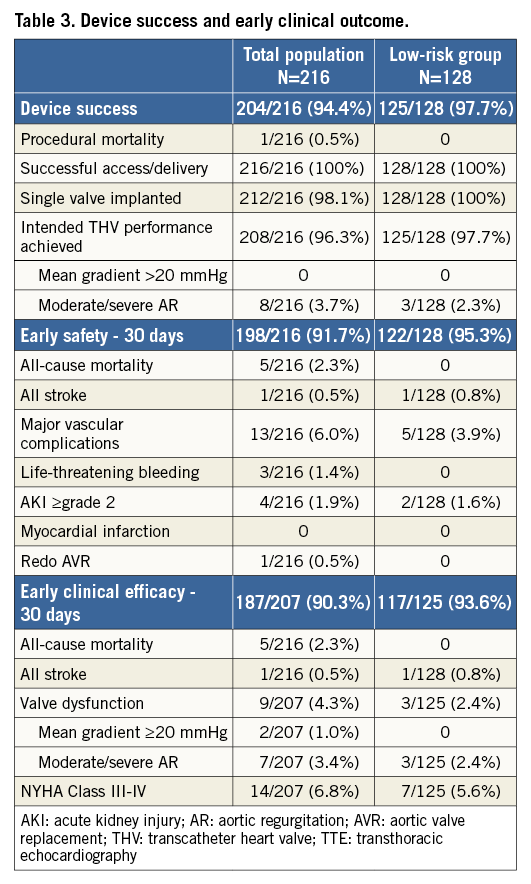
A permanent PM was implanted in 31 patients, corresponding to 15.8% of those patients “at risk” for PM (Table 2). Third-degree AV block was the most frequent indication (n=24) for PM implantation; other indications were second-degree AV block (n=2), bradycardia <40 beats per minute (n=2), first-degree AV block with bundle branch block (BBB) (n=2), and nodal rhythm (n=1).
Cardiac tamponade occurred in four patients. This was due to right ventricular (RV) perforation by a temporary pacing wire (two cases), left ventricular (LV) perforation by a stiff guidewire (one case), and annular rupture following post-dilatation (one case). The patient with a balloon-induced annulus rupture – creating a fistula between the aorta and RV outflow tract – was successfully converted to surgical aortic valve replacement (SAVR). A single procedural death occurred associated with the LV guidewire perforation (Table 2).
VALVULAR FUNCTION
At 30 days after TAVI, valvular function had improved compared with baseline. The aortic valve area (AVA) increased from 0.7±0.2 cm2 to 1.9±0.4 cm2 and the mean aortic valve gradient decreased from 46.5±14.1 mmHg to 7.4±3.8 mmHg (p<0.01) (Figure 2A). At 30 days, more-than-mild PVL was observed in 3.4% of all assessed patients (Figure 2C). These haemodynamic improvements persisted over time with an AVA at one year of 1.7±0.3 cm2 and a more-than-mild PVL in 4.5% of the assessed patients. Similar results were obtained in the low-risk group (Figure 2B, Figure 2D).
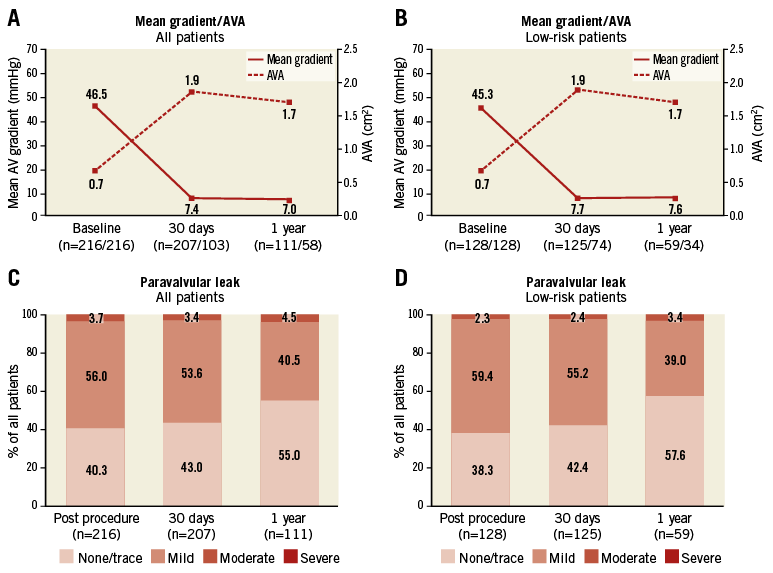
Figure 2. Valve function. Mean transvalvular gradient and AVA at baseline, 30 days and 1 year after TAVI for the entire study cohort (A) and the low surgical risk group (B). Paravalvular leak (PVL) immediately post procedure, at 30 days and 1 year after TAVI for the entire study cohort (C) and the low surgical risk group (D), as assessed by transthoracic echocardiography. Numbers of patients included in the assessment are indicated along the horizontal axis. AVA: aortic valve area; PVL: paravalvular leak
CLINICAL FOLLOW-UP
During the 30-day post-TAVI period, five patients died (2.3%), and stroke was reported in one patient (0.5%). Clinical follow-up data were obtained from all 211 patients alive at the 30-day assessment, with TTE and NYHA assessment in a subgroup of patients. Early safety was achieved in 198 patients (91.7%) and early clinical efficacy was achieved in 187 of 207 fully assessed patients (90.3%). Within the subgroup of 128 patients with low surgical risk, no deaths and one stroke occurred within 30 days post TAVI. Early safety was achieved in 95.3% of the patients in this subgroup (Table 3).
With follow-up of this registry ongoing, 24 patients died and four patients had a stroke within one year after TAVI. Kaplan-Meier estimates of one-year incidence rates for all-cause mortality and stroke were 12.3% and 2.3%, respectively (Figure 3).
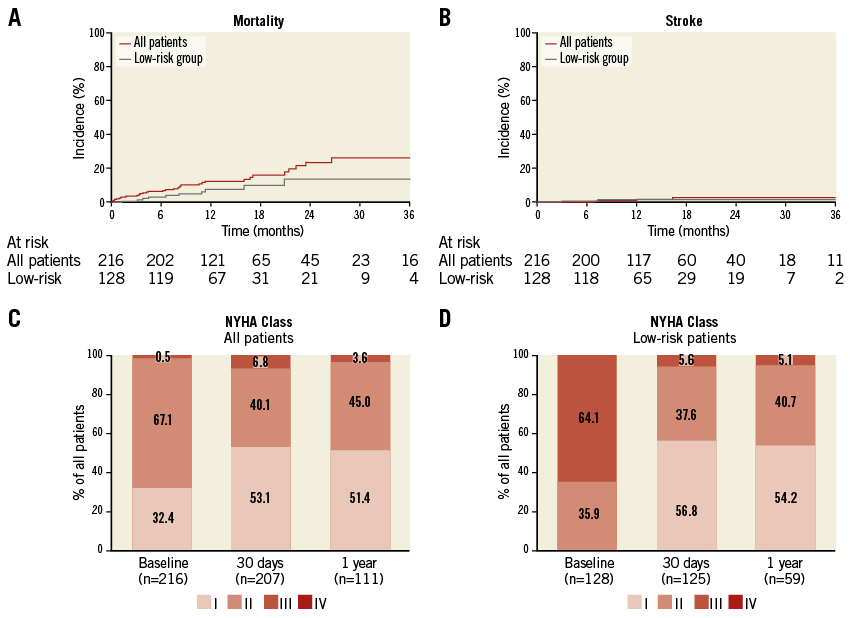
Figure 3. Clinical outcomes and follow-up. Kaplan-Meier cumulative incidence curves for all-cause mortality (A) and stroke (B) in the entire study cohort (red line) and the low surgical risk group (grey line). Functional NYHA class at baseline, 30 days and 1 year after TAVI for the entire study cohort (C) and the low surgical risk group (D); numbers of patients included in the assessment are indicated along the horizontal axis.
TEMPORAL CHANGES IN PROCEDURE-RELATED OUTCOMES
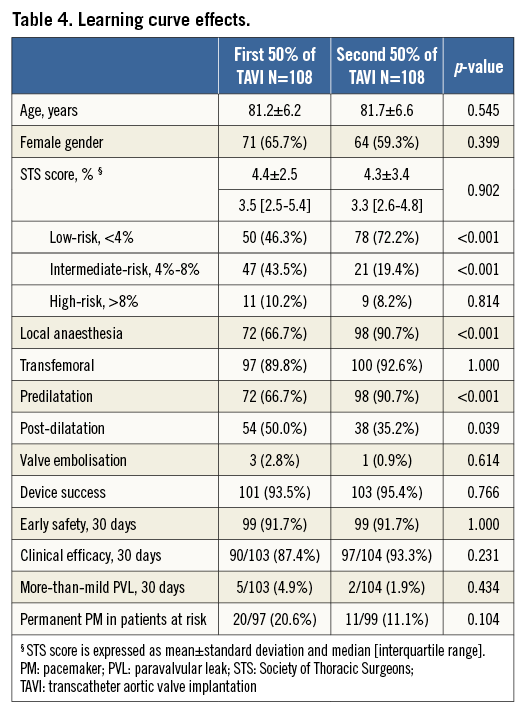
The data from this cohort represent our initial and ongoing experience with the Portico device. Outcomes from the first and second 50% of procedures are compared in Table 4. No marked differences were found in early safety and clinical efficacy at 30 days. Nevertheless, valve embolisations and permanent PM implantations were more frequent in the first 50% of procedures as compared to later procedures; however, statistical significance was not reached due to the small sample size. Over time, predilatation was applied more frequently (66.7% vs. 90.7%, p<0.01), and the incidence of more-than-mild PVL decreased from 4.9% in the early experience to 1.9% in the later experience cases.
Discussion
To our knowledge, this study comprises the first large real-life cohort implanted with the Portico device in an all-comers lower-risk patient population with one-year follow-up. In brief, the results indicate that implantation of the Portico valve was safe and associated with good clinical and haemodynamic outcomes up to one year of follow-up.
MORTALITY AND STROKE
Among the 216 procedures, there was a single procedural death, associated with LV perforation by a stiff guidewire and concomitant pericardial bleeding. Including this procedural event, there were five deaths and one stroke within 30 days after TAVI. Kaplan-Meier estimates at 30 days and one year were 2.3% and 12.3% for all-cause mortality and 0.5% and 2.3% for stroke, respectively. These outcomes are in line with and even better than mortality and stroke rates reported in other Portico studies4-8. However, comparison among these studies is difficult as the risk profile of these populations is often different (Table 5).
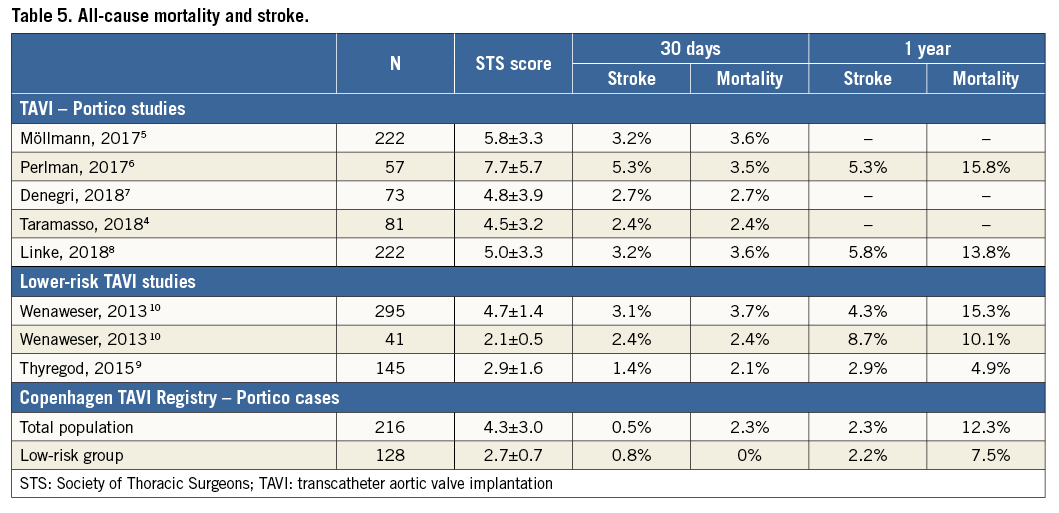
Almost 60% of the patients had a low operative risk (STS score <4%). Within the low-risk subgroup of the cohort, adequate outcomes were achieved in terms of mortality, stroke, early safety and clinical efficacy. Consistent with our results, the NOTION trial reported a 30-day all-cause mortality of 2.1% in a TAVI population with a mean STS score of 2.9%9. Another observational study reported 2.4% all-cause mortality at 30 days in a relatively small subgroup of patients with STS score <3%10. Overall, these findings suggest that TAVI can be safely performed in patients with a low surgical risk profile and warrant further research among this specific population.
HAEMODYNAMIC FINDINGS
Early haemodynamic performance was improved compared with baseline, with a low prosthetic valve gradient and increased valve area at 30 days after TAVI with the Portico valve. These improvements persisted over time, as shown in the patients with a one-year follow-up TTE (Figure 2). There were no cases of early valve failure or degeneration.
An important observation is the low incidence of more-than-mild PVL (3.4% and 4.5% at 30 days and one year, respectively). Other studies have confirmed that more-than-mild PVL is a rare finding with the Portico valve, with rates between 3.6% and 5.7%4-8. The option to resheath and reposition the valve and deploy the valve in a stable fashion may have contributed to these findings. In this cohort, the valve was repositioned in 31% of cases: this was because of initial suboptimal implantation depth or PVL. Other specific Portico features that may contribute to a low PVL rate are: 1) the large cells in the annulus section of the stent frame, which minimise the risk of annulus calcifications preventing complete stent frame expansion, and 2) the non-flared stent design, which helps to deploy the valve in a stable fashion and makes it less prone to dive into the LVOT at final release. Of note, post-dilatation was applied in 42.6% of the procedures, which is similar to other studies using this device; however, there was clearly more frequent use of predilatation in the second half of our patient cohort.
PM IMPLANTATION
Earlier reports indicated a relatively low rate of permanent PM implantation for the Portico valve, ranging from 9% to 15%4-8. Overall, 15.8% of all patients in the present cohort without prior PM required implantation of a permanent PM at 30 days post TAVI. The need to implant a permanent PM decreased over time to 11.1% in the second half of the cohort. This was calculated for the population “at risk” (i.e., excluding patients with prior PM). In comparison, rates of PM implantation for other TAVI devices in lower surgical risk populations are 8.3-9.9% for the ACURATE valve (Boston Scientific, Marlborough, MA, USA)11, 8.5% for SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) in PARTNER 212, 25.9% for CoreValve®/Evolut™ R (Medtronic, Minneapolis, MN, USA) in SURTAVI13, and 27.9% for the Lotus™ Valve System (Boston Scientific) in an intermediate-risk population14. Characteristics of the Portico valve that potentially reduce the risk of injury to the conduction system include the non-flared inflow section as well as the ability to reposition the device, facilitating valve implantation at optimal depth.
TEMPORAL CHANGES IN PROCEDURE-RELATED OUTCOMES
Our results do not clearly indicate a temporal change in composite clinical outcomes such as early safety and efficacy. Nevertheless, lower rates of valve embolisation, more-than-mild PVL and PM implantation were observed in later procedures as compared to the early period.
In fact, three of the four Portico valve embolisations at our centre occurred within the first 20 implantations. The reason for this was that the valve was released at too high a level (1-2 mm below the annulus) while not being fully expanded. Currently, predilatation is routinely performed in cases with more-than-mild calcifications and attention to full THV expansion before final release is something which is also taught to new operators. In the last 200 Portico cases in Copenhagen, there was only one single valve embolisation.
Over time, predilatation was more frequently applied (66.7% and 90.7% for first and second half of cases, respectively), an observation that also corresponds with a lower rate of more-than-mild PVL in later cases (4.9% vs. 1.9% for first and second half of cases, respectively). Predilatation seems to help in preventing non-uniform stent deployment and thereby facilitates gradual and slow THV deployment. Given the relatively lower opening force of newer-generation resheathable devices, slow deployment may also allow the stent to self-expand optimally and adapt to the specific anatomy. Of interest, post-dilatation was also less needed in case of more frequent predilatation.
Possible explanations for the lower PM implantation rate in the second half of the cohort are the systematic use of smaller balloons for predilatation (matching the minimum annulus diameter instead of the perimeter-derived mean diameter in the early period), less need for partial THV opening in the LVOT because of more routine predilatation (this manoeuvre was sometimes used in the early days in case of incomplete THV expansion at the annulus level), and more optimal final implantation depths in the second cohort.
OUTLOOK
The reported learning curve effects suggest that operators need to familiarise themselves with the specific implant behaviour and device characteristics of the Portico valve, which are not exactly the same as with other self-expanding heart valves. The present study shows that, after overcoming the learning curve, the Portico valve can be used as a versatile bioprosthetic aortic valve and achieves adequate outcomes in a wide range of patients.
An important characteristic of the Portico valve is the intra-annular position of the valve leaflets. This enables the valve to start functioning at the initial stage of deployment. Hence, valve deployment can be performed rather slowly, which, in combination with predilatation, allows optimal adaptation of the stent to the anatomy. Slow valve deployment may be a major learning point for operators experienced with other self-expanding valves when using the Portico valve. Moreover, it may be expected that the intra-annular valve position will result in fewer problems with coronary access, especially after redo TAVI (TAVI-in-TAVI) procedures. As TAVI indications are likely to be expanded to lower-risk and younger populations, this may become an important aspect.
Limitations
This study was performed as a real-world registry, with inherent limitations regarding patient selection – patients were selected for implantation with the Portico system at the operator’s discretion. Increasing experience with the implant may have influenced patient selection, resulting in improved outcomes over time. Data collection at one-year follow-up was not complete, which may cause bias, and there was no independent adjudication of complications and efficacy outcomes. Nevertheless, the study recruited consecutive patients and used established criteria for evaluation of safety and efficacy.
Conclusions
Implantation of the Portico aortic bioprosthesis in this lower surgical risk cohort was safe and associated with a high device success rate and low rates of PVL and PM implantation, especially after overcoming a learning curve effect. Moreover, the haemodynamic improvements achieved persisted over time.
| Impact on daily practice The Portico valve is a new-generation, resheathable transcatheter aortic valve that is safe to implant and that provides good outcomes with respect to haemodynamic performance, paravalvular leak (PVL) and permanent pacemaker implantation rate. Predilatation may help uniform valve deployment and reduction of PVL. |
Conflict of interest statement
L. Søndergaard has been a consultant for and received institutional research grants from Abbott Vascular, USA. O. De Backer and G. Bieliauskas have been consultants for Abbott Vascular, USA. The other authors have no conflicts of interest to declare.

