
Abstract
Aims: The aim of this study was to examine the short- and medium-term outcomes of transcatheter aortic valve replacement (TAVR) with the self-expanding and repositionable Portico valve (St. Jude Medical, St. Paul, MN, USA).
Methods and results: A total of 57 patients underwent TAVR with the Portico valve between March 2012 and August 2014, representing the first-in-human experience and the entire early experience in Canada. Patients were followed up at 30 days and one year with repeat echocardiography and clinical review. Patients were 80.8±7.3 years of age, and the Society of Thoracic Surgeons predicted risk of mortality was 7.7±5.7%. All patients had a valve implanted and four patients (7%) required a second valve. At 30 days, there were two deaths (3.5%), three disabling strokes (5.3%), and new pacemakers in five (8.8%) patients. Echocardiography revealed moderate/severe aortic regurgitation in two patients (3.6%). At one year, survival was 84.2% and echocardiographic findings were unchanged.
Conclusions: Transcatheter aortic valve replacement with the repositionable Portico valve provides satisfactory short- and medium-term haemodynamic and clinical results.
Abbreviations
AR: aortic regurgitation
AS: aortic stenosis
MDCT: multi-detector computed tomography
NYHA: New York Heart Association
TAVR: transcatheter aortic valve replacement
TEE: transoesophageal echocardiography
TTE: transthoracic echocardiography
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve replacement (TAVR) has proven to be a safe and effective option for many patients with severe symptomatic aortic stenosis (AS)1,2. Newer transcatheter heart valves have attempted to improve on the limitations of earlier systems. The ability to reposition, recapture and redeploy a partially or fully deployed valve may be particularly desirable when the initial implant positioning is suboptimal.
The Portico™ valve system (St. Jude Medical, St. Paul, MN, USA) is a transcatheter aortic valve consisting of a nitinol self-expanding frame containing three bovine pericardial leaflets and a porcine pericardial sealing cuff3. The outflow portion of the stent frame incorporates three retention tabs, which secure the crimped valve to the delivery system during most of the deployment process. There are four sizes of Portico valve, measuring 23 mm, 25 mm, 27 mm and 29 mm at the inflow level. The catheter incorporates a soft tapered nose cone and an 18 Fr capsule that contains the compressed valve. The ability to retrieve and to reposition the Portico valve represents two important advantageous features of this new valve system.
Whilst preliminary clinical results using the Portico valve have been positive4,5, there have been limited reports of non-transfemoral delivery6 and longer-term clinical follow-up. Furthermore, the temporary halt of international Portico implantations due to reports of early reduced leaflet mobility (presumably due to thrombus) raised possible safety issues with the device. We therefore aimed to report short- and medium-term clinical and haemodynamic outcomes of the Portico valve to determine its relative safety and efficacy further in a real-life setting.
Methods
A total of 57 patients representing the first-in-human experience and entire early Canadian experience with Portico valves were included. Multimodality assessment and eligibility decisions were conducted in each of four centres by a multidisciplinary team. Patients were eligible for inclusion if they had severe symptomatic aortic valve stenosis and were judged to be at high surgical risk.
Patients were assessed in a comprehensive manner, including clinical evaluation, transthoracic echocardiography, multi-detector computed tomography (MDCT) and invasive angiography. MDCT examinations were performed and interpreted according to recommended criteria7. Prosthesis sizing was determined on the basis of aortic annulus measurements as previously described3,8. Prostheses sizes 27 mm and 29 mm became available for use at the beginning of 2014; this was after 30 (52.7%) patients had been treated. The implantation procedure has been explained previously9,10. The procedures were guided by fluoroscopy/angiography±transoesophageal echocardiography (TEE). Figure 1 depicts a fluoroscopic image of an implanted Portico valve. Post-TAVR antiplatelet treatment with aspirin and clopidogrel was given to most patients; however, there was great variability in treatment protocols that depended on previous coronary interventions, anticoagulation status and other factors. Procedural data, 30-day and 12-month clinical events were prospectively recorded and defined according to the Valve Academic Research Consortium-2 (VARC-2) criteria11. All patients underwent a complete transthoracic echocardiographic (TTE) examination, according to the guidelines of the American Society of Echocardiography12,13, before the procedure and within 30 days post TAVR and at one year. The presence, degree, and type (paravalvular vs. transvalvular) of aortic regurgitation (AR) was reported at each participating site. The AR severity was evaluated using a multi-parametric approach and classified following the VARC-2 recommendations. All TAVR procedures were performed under a compassionate clinical use programme approved by Health Canada, and all patients provided signed informed consent.
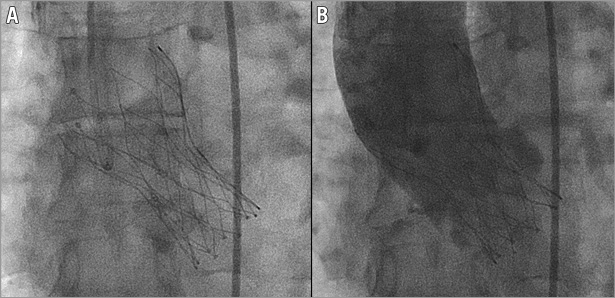
Figure 1. A Portico valve after deployment in the aortic valve. Fluoroscopic appearance of a 23 mm Portico valve before (A) and after (B) contrast injection in the aortic root.
DEFINITIONS AND ENDPOINTS
The primary study endpoint was all-cause mortality at 30 days of follow-up. Secondary outcome measures included in-hospital major adverse cardiac events, including all-cause mortality, cerebrovascular events, myocardial infarction, bleeding complications, vascular or access-related complications and acute kidney injury. Longer-term follow-up and outcomes at one year were reported in surviving patients. Serious adverse events and other outcomes were site reported. All events were coded according to the standardised endpoint definitions proposed by the VARC-2 guidelines.
STATISTICS
Descriptive summaries are reported for all TAVR cases, as well as separately by access site. Baseline characteristics, procedural details and clinical outcomes are reported as counts and percentages for categorical variables, and median and standard deviations (SD) for continuous variables. All analyses were carried out using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 57 patients underwent TAVR with the Portico valve at four sites in Canada between March 2012 and August 2014. Baseline characteristics are described in Table 1. The mean age was 80.8±7.3 years and 82.5% of patients were female. Patients were symptomatic, with 75.4% in NYHA III or IV functional class. Patients had severe aortic stenosis with an average aortic valve area of 0.65±0.22 cm2 and a mean transvalvular gradient of 41.8±16.0 mmHg.
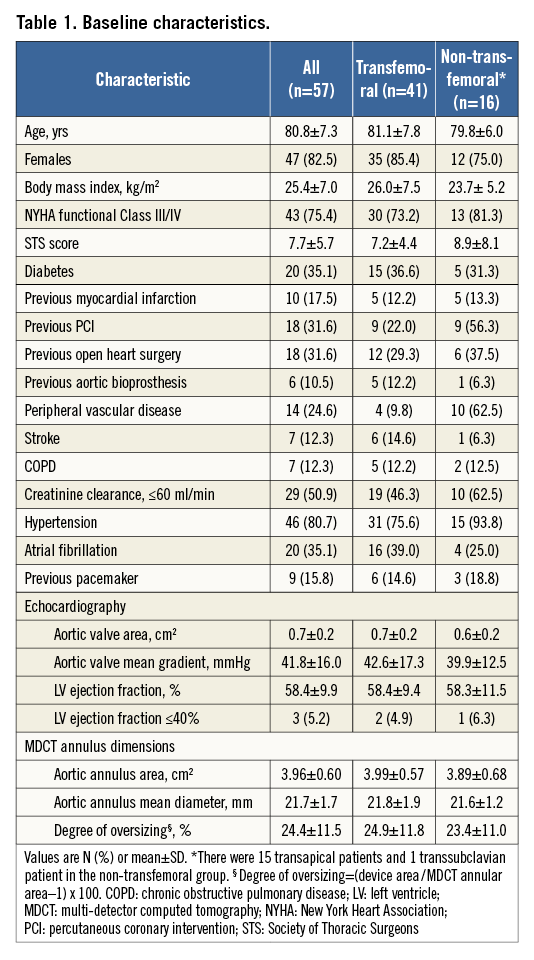
Procedural details are highlighted in Table 2. Transfemoral access was performed in 41 (71.9%) cases, apical access in 15 (26.3%) patients and subclavian access in one (1.8%) patient. VARC-2-defined procedural success was achieved in 43 (75.4%) cases. A second transcatheter valve was required in four (7.0%) transfemoral patients: two patients required a second valve after the first valve was deployed too high into the aorta, above the annulus, one patient had severe transvalvular regurgitation secondary to an immobile (“frozen”) leaflet, and one patient had severe paravalvular regurgitation secondary to a very low deployment position. Post-dilation was performed in nine (15.8%) patients to reduce paravalvular regurgitation.
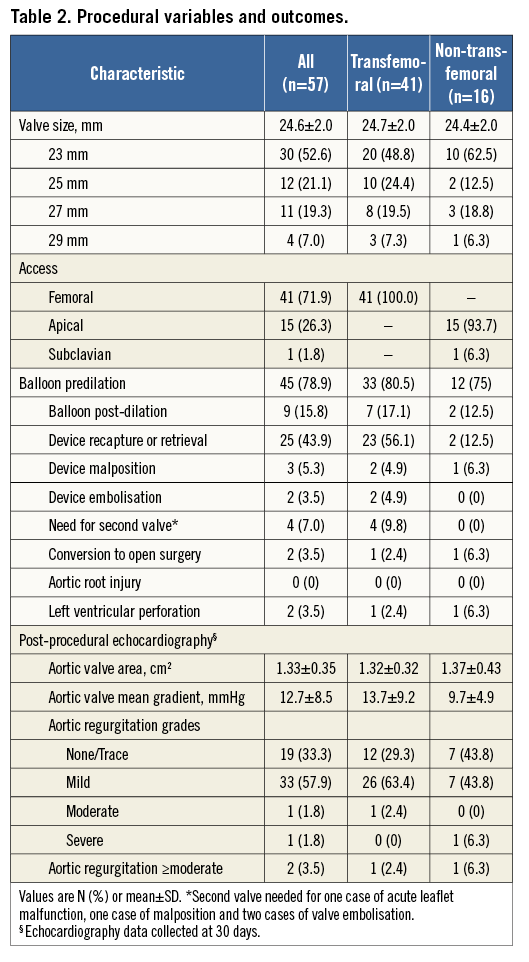
Patient outcomes are presented in Table 3. All-cause 30-day mortality occurred in two (3.5%) patients: both were transapical periprocedural deaths. Disabling stroke occurred in three (5.3%) patients and no (0%) patients had a periprocedural myocardial infarction. Major vascular complications occurred in five (8.8%) patients, and seven (12.3%) patients experienced life-threatening or major bleeding complications. New permanent pacemaker implantation was required in five patients (8.8%, representing 10.4% of patients without previous pacemakers). Analysis of patients according to access site revealed numerically lower procedural and 30-day mortality (0% vs. 12.5%, p=0.07), strokes (4.9% vs. 12.5%, p=0.31) and life-threatening bleeding (2.4% vs. 12.5%, p=0.19) in transfemoral cases compared to non-transfemoral cases, respectively.
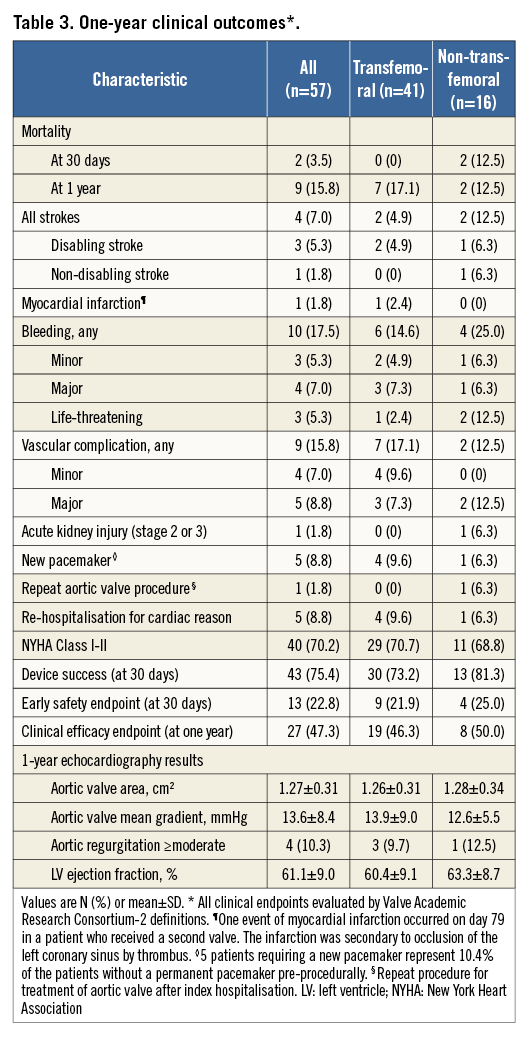
At one-year clinical follow-up (Table 3) survival was 84.2%. One patient, who had two overlapping valves implanted, died at two months due to thrombosis of the coronary sinus and resultant left main occlusion (Figure 2). A second patient with two implanted transcatheter valves, who also had a new pacemaker implanted after TAVR, died suddenly at home five months after TAVR. There were seven additional deaths in the first year, five of which had a cardiovascular cause. At one year, one patient suffered a late stroke (a patient with atrial fibrillation who had also suffered a major periprocedural stroke), and four (8.1%) patients were re-hospitalised for heart failure.
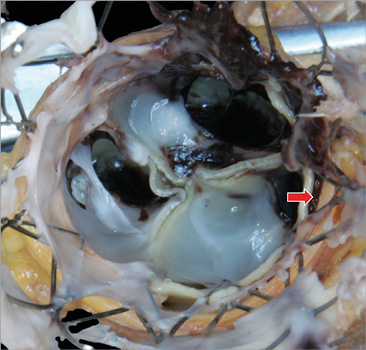
Figure 2. Left main occlusion with thrombus in a patient with two implanted valves. Post-mortem evaluation of the aortic root of a patient who died of left main occlusion and myocardial infarction. There are two overlapping Portico valves and a thrombus is identified on the outer side of the valves. The thrombus (red arrow) extends above and over the sealing skirt of the inner valve, thus filling the sinus adjacent to the left main artery and occluding blood flow into the vessel.
Echocardiographic data post TAVR, at 30 days and one year are listed in Table 2 and Table 3. At 30 days, the overall mean aortic gradient decreased from 42±16 mmHg to 13±9 mmHg, and the mean aortic valve area increased from 0.65±0.22 cm2 to 1.33±0.35 cm2 post TAVR. At 30 days, paravalvular AR was absent or trivial in 32.7% of patients, mild in 58.1%, moderate in 1.8% and severe in 1.8%.
Follow-up echocardiography at one year post implant was available in 39 patients (81.3% of survivors), with stable mean aortic valve gradients and aortic valve areas maintained up to 12 months (Figure 3). Four (10.3%) patients had moderate or worse paravalvular AR at one year of follow-up.
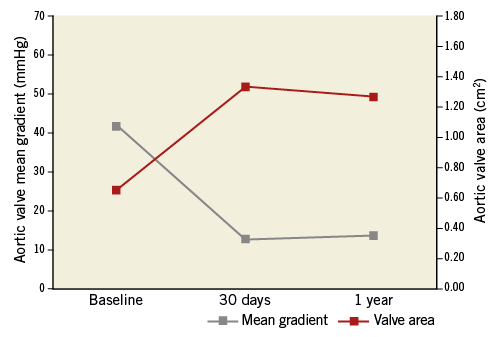
Figure 3. One-year changes in aortic valve mean gradient and area. Aortic valve mean gradients and valve areas are shown at baseline, 30 days and one year after implantation of a Portico valve.
Interestingly, on a routine transthoracic echocardiogram one patient was noted to have a thrombus present on the valve at one year. The patient was asymptomatic and the mean gradient (6 mmHg) and paravalvular regurgitation (trivial) were unaffected.
Discussion
This study was a multicentre countrywide evaluation of the short- and intermediate-term outcomes of patients undergoing TAVR with the Portico valve. The main findings include satisfactory 30-day and one-year mortality and safety outcomes, which are favourable compared to other transcatheter valve technologies.
Whilst TAVR appears to be a transformative technology for patients with severe symptomatic AS, a number of limitations remain with current transcatheter technologies. These include stroke14, need for permanent pacemaker15, aortic root and vascular injury and paravalvular AR16.
The overall 30-day mortality rate of 3.5% in high-risk patients undergoing TAVR with the Portico valve is comparable to recent international registry outcomes utilising the two most common TAVR platforms, the Edwards SAPIEN balloon-expandable valve (Edwards Lifesciences, Irvine, CA, USA) and the self-expanding CoreValve® (Medtronic) device17-20.
In this initial series of 57 patients, 72% of the procedures were performed via the transfemoral approach with no 30-day mortality (0%). Whilst small numbers prevent direct comparisons, our study indicated a higher mortality of 12.5% with the non-transfemoral approach (mostly by the transapical route). The Portico transapical delivery system requires, for unsheathing of the valve, that the encasement capsule be advanced across the aortic arch. This manoeuvre, at times, required a significant amount of force and tension to be applied on the delivery system and on the apical access site. This resulted in one case of left ventricular laceration from which the patient did not recover. The second patient died from a massive bleed in the left hemithorax on day three, possibly also related to the access site. In addition, patients requiring non-femoral access often represent a higher surgical risk group, as was seen in this cohort. Numerous reports of both balloon-expandable and self-expanding transcatheter valves have similarly highlighted increased mortality in patients utilising the non-transfemoral approach, presumably related in part to the higher risk profile of the patients18-22.
Our reported low rate of moderate or severe paravalvular leaks of 3.6% at 30 days is similar to other early reports of the Portico valve4,5, and compares favourably to reports with the self-expanding CoreValve system16,23. Further confirmation of the low rate of paravalvular AR is given by the low rate (15.8%) of post-dilation performed in this study of the self-expanding Portico valve.
Additionally, our study highlights the utility of a repositionable/retrievable transcatheter aortic system, which may contribute to an improved final positioning of the valve, a key determinant of valve performance in self-expanding transcatheter platforms, reducing rates of paravalvular AR and the need for permanent pacemaker implantation24. The low rate of new pacemaker implantation in our cohort (8.8%), comparable to results in balloon-expandable valves, may be explained by the ability to position the valve accurately or possibly by the lower radial forces of the Portico valve. The Portico valve is repositionable and retrievable until about 80% of the unsheathing process of the valve. The final deployment of the valve may result, because of tension forces released at this stage when the valve is still not well anchored, in its displacement, when no longer retrievable. This process explains the disappointing occurrence of three cases of severe paravalvular AR after the final deployment of the first valve that necessitated the use of a second valve. A fourth patient required a second valve because of a technical failure of one of the leaflets of the first valve. Two of these four patients died within a few months of the procedure. In one case, this was directly related to obstruction of the left main artery by a thrombus that formed in the left coronary sinus. The reason for this was probably reduced flow related to the proximity of the valve stents and tissue to the left main orifice. A review of patients who required second valve implants suggests that mortality is higher in these cases, and this is more common in the early experience of centres25, as was the case in this study of the early Canadian experience with the Portico valve.
The reported aortic valve area after TAVR in our cohort is relatively small, as compared with other transcatheter valves26,27. Incomplete expansion cannot be ruled out; however, this may be due in part to a limited availability of larger valve sizes in this early experience; more than 50% of the valves used were of the smallest size available (23 mm).
St. Jude Medical temporarily halted the Portico valve programme in September 2014 following the detection of reduced valve leaflet mobility, as evaluated by dynamic ECG synchronised cardiac computed tomography. Further analysis has revealed that reduced valve mobility was probably related to early thrombus formation, something which has also been documented across other transcatheter and surgical aortic valve platforms28. We similarly report one case of valve thrombosis at one year, which did not have clinical or haemodynamic significance. At this time, reports of suspected thrombi identified on Portico valves and other valve types are of unclear clinical relevance and require further follow-up.
Study limitations
This is a small multicentre observational cohort not powered for analysis of predictors of outcomes with the Portico transcatheter heart valve. Furthermore, the patients represent the early experience of the Canadian centres, possibly contributing to less optimal results. Data, including patient and procedural characteristics, and outcomes are reported by each site, without adjudication or central core lab analysis.
Conclusions
Satisfactory 30-day and one-year clinical outcomes were demonstrated in high-risk patients treated with the Portico transcatheter heart valve. Confirmation of these early findings and further experience with non-transfemoral cases are needed with ongoing randomised trials and clinical registries.
| Impact on daily practice The Portico valve is a new repositionable and retrievable aortic valve device that has the ability to deliver good clinical results regarding paravalvular AR and the need for pacemakers. The Portico valve, especially when delivered by the transfemoral route, is associated with very good 30-day safety outcomes. Clinicians should be aware of the possibility of malpositioning the valve in the final, non-retrievable stage of the deployment. |
Conflict of interest statement
J. Webb reports being a consultant to St. Jude Medical. J. Leipsic, D. Wood and D. Dvir report being consultants to Edwards Lifesciences. The other authors have no conflicts of interest to declare.

