
Introduction
Among anatomical factors affecting transcatheter aortic valve implantation (TAVI) success, aortic annulus shape plays a relevant role: an elliptic geometry may lead to gaps between the aortic root and the prosthesis, which is designed to expand circularly, resulting in paravalvular leak (PVL). In this context, the impact of different self-expanding (SE) devices is currently unknown.
The aim of the study was to compare PVL and device success rates of the Portico and Evolut R in patients with an elliptic aortic annulus and to test by computational simulations their behaviour by increasing eccentricity.
Methods
This retrospective study included 374 patients with symptomatic severe aortic stenosis undergoing TAVI with the Portico™ (Abbott Vascular, Santa Clara, CA, USA) and Evolut™ R (Medtronic, Minneapolis, MN, USA) valves and with available computed tomography (CT) angiography measurements of the aortic annulus (Supplementary Figure 1).
The index of eccentricity (IE) was calculated as (1–short-axis/long-axis annulus diameter); IE >0.25 defined an elliptic aortic annulus1.
Post-procedural PVL was assessed with transthoracic echocardiography and classified into four categories (absent/trivial, mild, moderate, severe) by experienced echocardiographers who were blinded to the aortic annulus measurements2.
Device success was defined according to the VARC-2 definition2.
Geometrical models of the Evolut R and Portico were reconstructed from micro-CT scans of real device samples (Evolut R 26 mm and Portico 25 mm) and nitinol material properties were assigned (Supplementary Figure 2); an idealised model of the aortic root was conceived with increasing eccentricities (0/0.25/0.5) and used for finite element simulation of TAVI3 (Supplementary Figure 3). Post-processing of simulation outcomes was performed to compute stent-root interaction area, von Mises stress distribution of the LVOT/annulus region, and paravalvular orifice area. Statistical methods are detailed in Supplementary Appendix 1.
Results
Of 374 patients, 107 (28.6%) had an elliptic annulus and were more frequently women (Table 1). Elliptic annulus patients were equally distributed in the Portico and Evolut R groups (25.2% vs 30.0%; p=0.35); among them, oversizing was greater in the Evolut R than in the Portico group, whereas the predilatation rate and implantation depth were overall higher in Portico patients (Table 2). Postoperatively, ≥moderate PVL rate was similar between Portico and Evolut R patients (7.2% vs 8.4%; p=0.71). Higher rates of ≥moderate PVL were observed in the elliptic group only in Evolut R patients (Table 2). Device success was comparable between Portico and Evolut R patients (91.9% vs 90.5%; p=0.67). Causes of device failure were ≥moderate PVL in 30 (88.2%) patients and implantation of a second prosthesis in 4 (11.8%) patients.
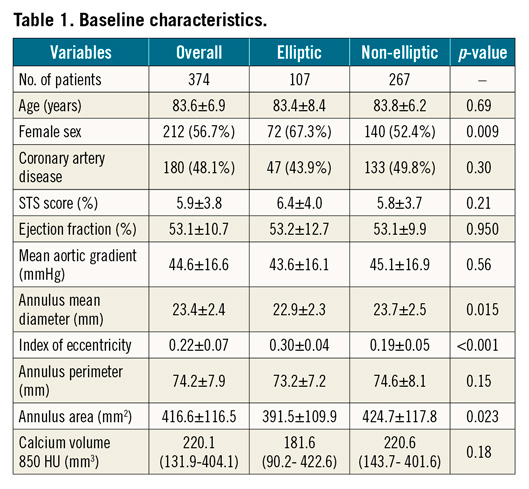
Among elliptic annulus patients, device success was higher in the Portico group than in the Evolut R group (Figure 1A). On the other hand, device success in the Evolut R elliptic group was lower compared to that of non-elliptic annulus patients (Figure 1A), mainly driven by a higher rate of ≥moderate PVL in the former (15.2% vs 5.4%; p=0.009).
On receiver operating characteristic (ROC) curve analysis, eccentricity was predictive of device success only among Portico patients (Figure 1B, Figure 1C).
On simulation analysis, the Portico device maintained substantially similar values of stent-root interaction area and symmetric patterns of stress distribution by increasing eccentricity from 0.25 to 0.5, whereas the Evolut R showed a sudden drop, resulting in a steep increase in paravalvular orifice area by IE=0.5 as compared to the Portico (Figure 1D-Figure 1F, Figure 2).
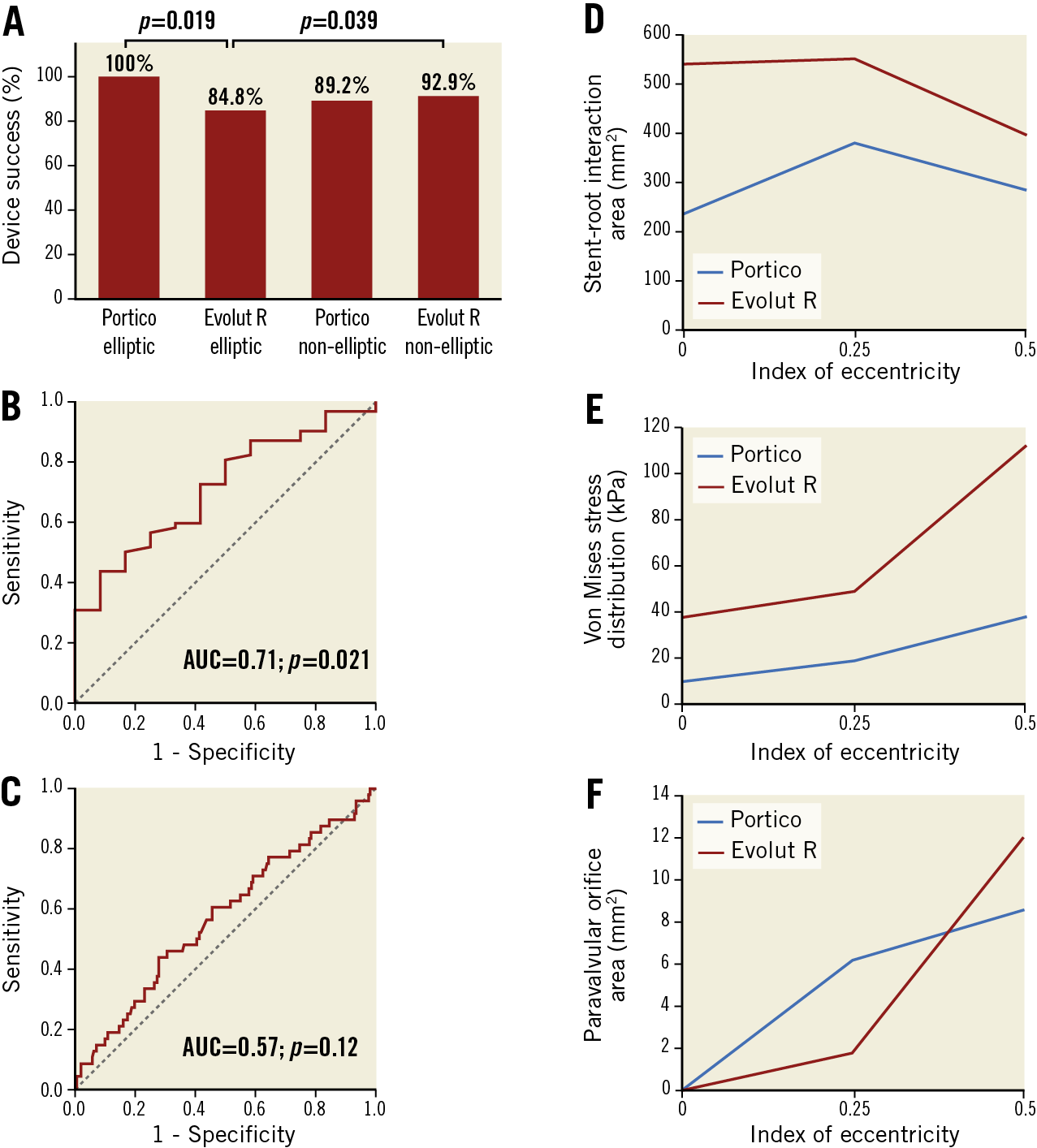
Figure 1. Device success and simulation post-processing. A) Device success rates; capability of the index of eccentricity to predict device success in the Portico (B) and device non-success in the Evolut R group (C). Stent-root interaction area (D), stress distribution (E) and paravalvular orifice area (F) of the Portico and Evolut R.
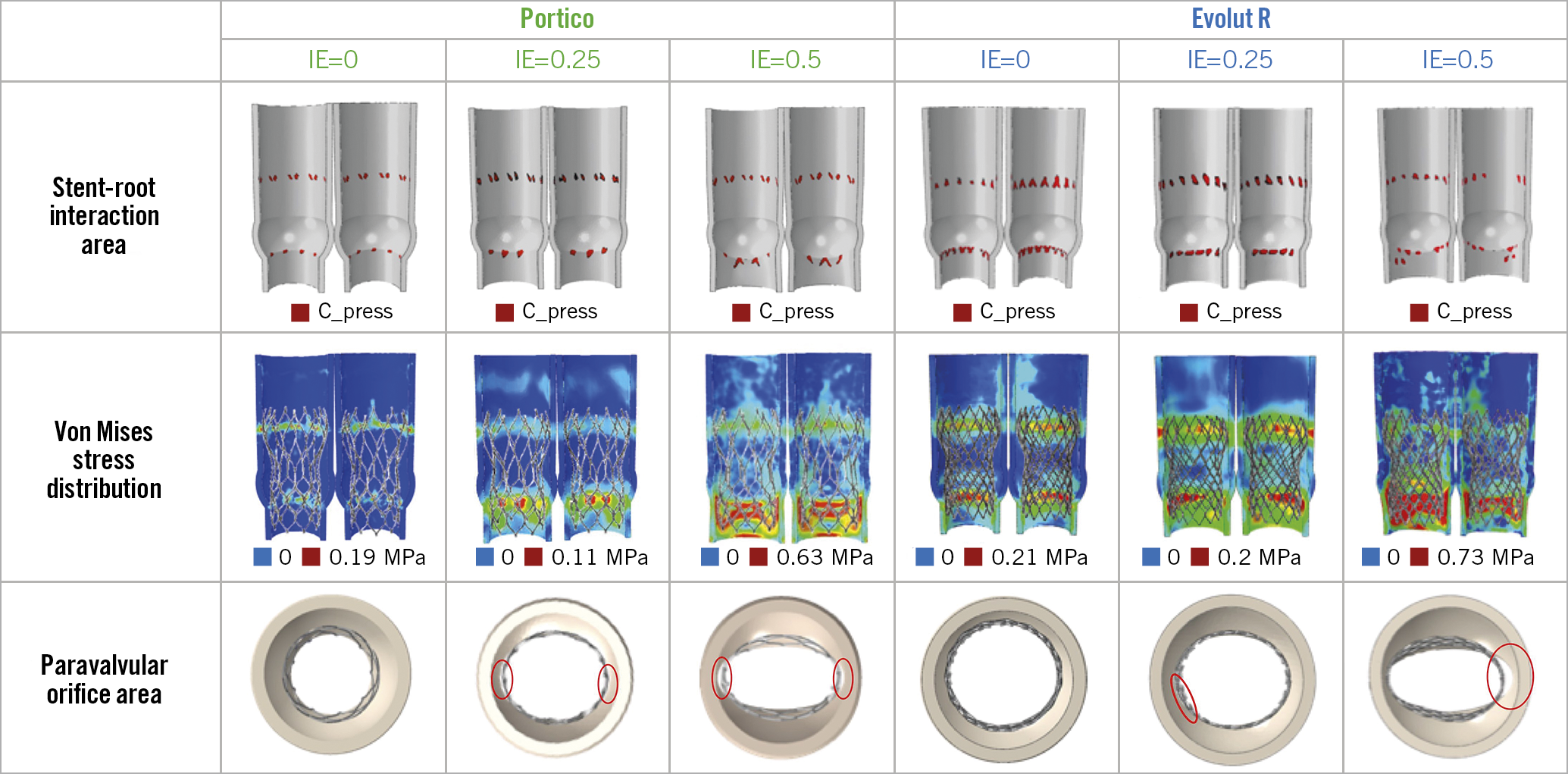
Figure 2. Simulation analysis.
Discussion
The issue of aortic annulus eccentricity has been investigated in only a few studies. Maeno et al reported higher rates of device success and lower rates of at least moderate PVL in elliptic annulus patients treated with balloon-expandable (BE) rather than SE devices. This is due to the higher radial force of BE valves which reshape the geometry of the aortic annulus from elliptic to circular, in contrast with the CoreValve® (Medtronic)4,5. However, in patients with very elliptic annuli (IE >0.30, 13.9% in our study), BE devices may injure the aortic root, whereas a highly conformable SE device may be safer and equally effective.
On computational simulation, the small paravalvular orifice area observed with the Portico in very elliptic annuli resulted from a better compliance of the stent frame to the aortic annulus, probably related to its cells which are larger than those of the Evolut R. Large cells reduce the radial force of the Portico, which is approximately one-third that of the Evolut R, but increase its conformability, providing optimal sealing when eccentricity is pronounced (Supplementary Figure 4, Supplementary Figure 5). Conversely, when eccentricity is mild (IE=0.25), the greater radial force of the Evolut R may compensate for its lower compliance.
Simulation findings seemed consistent with the clinical data with respect to ≥moderate PVL for Evolut R elliptic versus non-elliptic annulus patients and Portico versus Evolut R elliptic ones. Notably, the higher ≥moderate PVL rate in Portico non-elliptic versus elliptic annulus patients (9.6% vs 0.0%; p=0.20) may be due to the greater but still not significant calcium volume in this group, despite a similar implantation depth (Supplementary Table 1-Supplementary Table 4).
Prosthesis function was comparable between the Portico and Evolut R even by IE >0.30 (6.2±1.6 mmHg vs 7.5±3.9 mmHg; p=0.46) despite the intra-annular valve position of the Portico.
Limitations
The number of Portico and Evolut R patients was not perfectly balanced due to our institutional policy (Evolut R was used in half of the procedures) and to the 0.25 cut-off to define ellipticity in order to provide a better insight into an uncommon but still complex population1. Additionally, the low number of events prevented multivariate analysis.
Conclusions
In patients with an elliptic annulus, implantation of the Portico valve showed excellent ≥moderate PVL and device success rates, compared to the Evolut R. This may be due to the high conformability of the Portico to elliptic geometry.
Acknowledgements
The authors would like to thank Giovanni Bianchi, MSc, and Elena Acerbi, BSc, for their valuable help in data collection and editing of the database, and cath lab technicians for CT-angiography analysis.
|
Impact on daily practice In elliptic annulus patients, the Portico valve showed optimal conformability, lower ≥moderate PVL and higher device success rates than the Evolut R. |
Conflict of interest statement
F. Bedogni and F. De Marco are consultants for Medtronic and Abbott. N. Brambilla is a consultant for Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

