
Abstract
Aims: The aim of this study was to evaluate the safety and performance of the resheathable and repositionable St. Jude Medical Portico self-expanding transfemoral TAVI system.
Methods and results: This prospective, single-arm, multicentre study evaluated the 18 Fr Portico system with either a 23 or a 25 mm valve. Patient follow-up was at 30, 90, 180 days and one year. Results up to 30 days are presented. Adverse events were categorised by VARC definitions and adjudicated by an independent events committee. Echocardiography was evaluated by an independent laboratory and all patients underwent neurological assessment at baseline, post procedure, at 30 days and one year. The primary endpoint was all-cause mortality at 30 days. A total of 102 patients (mean age 84.1±4.8 years; 97% female; median STS score 5.6) were enrolled. The 30-day mortality, disabling stroke and major vascular complications were 2.9%, 2.9% and 5.9%, respectively. Resheathing and repositioning (23.8%) was successful in all instances. Paravalvular leak at 30 days was none/trace in 30.4%, mild in 65.8% and moderate in 3.8%. Mean gradient improved from 45.3±13.8 to 8.9±3.8 mmHg and valve area improved from 0.6±0.2 to 1.7±0.4 cm2. Permanent pacemaker implantation was required in 9.8% of patients.
Conclusions: The novel Portico TAVI system is safe and effective at treating high-risk patients with symptomatic severe aortic stenosis, allowing safe repositioning and optimisation of device position. ClinicalTrials.gov Identifier: NCT01493284
Abbreviations
AVR: aortic valve replacement
BAV: balloon aortic valvuloplasty
CEC: clinical events committee
PVL: paravalvular leak
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
TF: transfemoral
Introduction
Transcatheter aortic valve implantation (TAVI) is a recommended treatment option for patients deemed high-risk or at surgically prohibitive risk following assessment by the Heart Team1-4. Complications following TAVI, however, are still a concern. Some of these could be due to a suboptimally positioned valve5,6; a repositionable TAVI device may mitigate some of these complications.
The Portico™ 18 Fr TAVI system (St. Jude Medical, St. Paul, MN, USA) (Figure 1) was designed to be resheathable and repositionable. The device characteristics have been described in detail previously7. In brief, it is an annular functioning, trileaflet bovine pericardial valve, mounted on a self-expanding nitinol frame (Figure 1). The skirt is made from porcine pericardium and works synergistically with the widely spaced conformable nitinol frame to seal the native annulus and mitigate PVL.
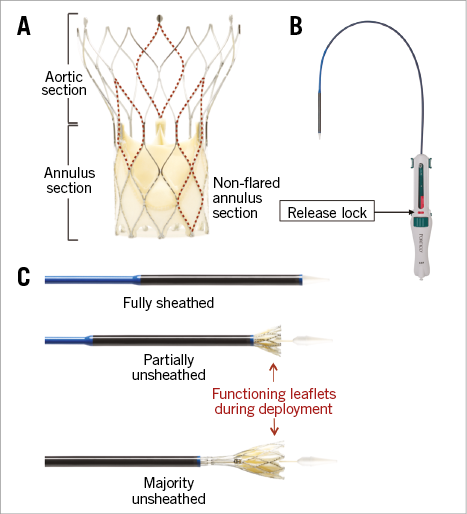
Figure 1. St. Jude Medical Portico TAVI system. A) The Portico valve is an annular functioning valve, with large cell frame enabling easy access to coronary arteries. B) The Portico delivery system is 18 Fr compatible. The “release lock” (red tab) protects from inadvertent release of the valve. C) Delivery system and valve at varying stages of release. The valve can be fully resheathed following 80% release.
The objectives of this study were to assess the safety and performance of the transfemoral (TF) Portico TAVI system in treating patients with symptomatic severe aortic stenosis who are at high or prohibitive surgical risk for aortic valve replacement (AVR). The 30-day outcomes are presented.
Methods
STUDY DESIGN
This multicentre, prospective, non-randomised study, without concurrent or matched controls, was designed to assess the 30-day all-cause mortality rate in patients treated with either the 23 or 25 mm Portico valve at six centres in the United Kingdom and Germany (Online Appendix). In addition, safety and performance data of the transfemoral delivery of the Portico transcatheter heart valve implant and delivery system were evaluated. The study was sponsored by St. Jude Medical.
All study source data were independently monitored for accuracy on site. An independent clinical events committee (CEC) reviewed all reported events. All study site echocardiographers were trained on a standardised echocardiogram acquisition protocol for the study, and an echocardiography core laboratory (Online Appendix) evaluated all echocardiograms and echocardiographic study endpoint results.
This study was conducted in accordance with the Declaration of Helsinki. Local ethics committee approval, along with applicable local regulatory requirements, was obtained, and all patients who met the study inclusion and exclusion criteria (ClinicalTrials.gov Identifier: NCT01493284) signed an informed consent prior to enrolment and the performance of any study-related investigations.
PATIENT SELECTION AND PROCEDURAL CONSIDERATIONS
Patients at high or prohibitive surgical risk with symptomatic (NYHA II or greater) severe aortic stenosis, defined by echocardiography-derived mean gradient >40 mmHg or jet velocity greater than 4.0 m/s or an initial valve area of <1.0 cm² (or aortic valve area index ≤0.6 cm²/m²) were considered for the study.
All suitable patients were initially assessed by each institution’s Heart Team for risk assessment, followed by a subject selection committee review, whose role was to ensure that the patient met all of the inclusion criteria and none of the exclusion criteria. Key exclusion criteria included patients with a native aortic valve that was congenitally unicuspid, bicuspid, quadricuspid or non-calcified as seen by echocardiography, patients with mitral or tricuspid valvular regurgitation (>grade III) or moderate to severe mitral stenosis and patients with left ventricular ejection fraction <20%.
As per protocol, only transfemoral access was allowed. The annulus diameter and perimeter requirements for the two valve sizes were: 23 mm=19-21 mm diameter and 60-66 mm perimeter; 25 mm=21-23 mm diameter and 66-73 mm perimeter.
Following crossing of the native aortic valve, a pre-shaped stiff support wire was positioned in the left ventricular cavity. Pre-TAVI balloon aortic valvuloplasty (BAV) was recommended in all patients. Patient ECGs were sampled at key procedural steps (pre-procedure, on crossing the valve, pre-BAV, pre-TAVI and post procedure).
Resheathing and repositioning was recommended if the intended depth of implant (1-9 mm) was suboptimal or if the valve migrated to the ascending aorta during release or if PVL was observed. A contrast-enhanced aortogram was recommended to confirm valve position and coronary perfusion before final valve release. Inadvertent valve release is mitigated by the “release lock” mechanism (Figure 1).
All patients were followed up clinically, with echocardiography at baseline, pre-discharge, 30 days, three months, six months, and one year. Neurological assessments by a neurologist or trained neurological physician were conducted at baseline, pre-discharge, 30 days, and one year, and at 90 days following any neurological event. Post-procedure anticoagulation or antiplatelet regime was at the discretion of the implanting team and was based on the relative risk of bleeding to the patient.
STUDY ENDPOINTS
The primary endpoint of the study was all-cause mortality at 30 days. The secondary endpoints are summarised in Table 1. The Valve Academic Research Consortium (VARC)8 definitions were used by the clinical events committee for adjudication of the endpoint events.
STATISTICAL METHODS
Continuous variables are summarised using mean±standard deviation (SD) or median and interquartile range (IQR). Normality of data was verified with the use of the Kolmogorov-Smirnov normality test. Categorical variables are summarised using frequencies and percentages. The paired t-test was used to compare aortic mean gradient and valve area between baseline and post TAVI at 30 days. Statistical significance was achieved if a two-sided test obtained a p-value <0.05. SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
BASELINE
A total of 100 patients (mean age 84.1±4.8 years; 97.1% female), with 50 patients in each valve size, were treated with the Portico TAVI system (Table 2). Two patients had an attempted procedure, where a delivery system entered the patient but a valve was not successfully implanted (one patient received a commercially available valve and in the other patient there was difficulty advancing the 18 Fr sheath and the procedure was stopped). These subjects were followed up to 30 days and are included in the 30-day analysis (total 102 patients for analysis).
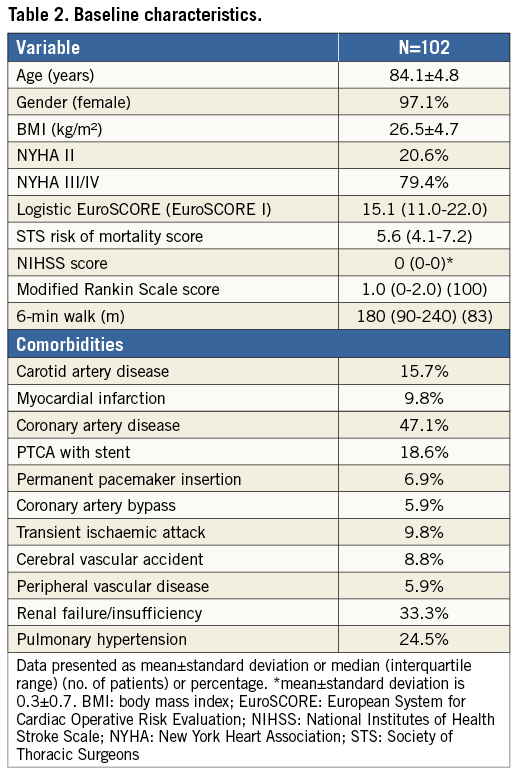
The majority of patients had NYHA Class III or IV symptoms (79.4%), with 47.1% having underlying coronary artery disease and 33.3% renal insufficiency. Pre-existing cardiac arrhythmia was present in 52% of patients, with 54.7% of these in atrial fibrillation. The median Society of Thoracic Surgeons (STS) score and logistic EuroSCORE I were 5.6 and 15.1, respectively (Table 2).
PROCEDURAL OUTCOMES
The majority of patients had TAVI under local or conscious sedation (65.7%) (Table 3). None of these required conversion to general anaesthesia. All patients underwent balloon predilatation. Cardiopulmonary bypass was required in one patient due to left ventricular perforation requiring surgical intervention.
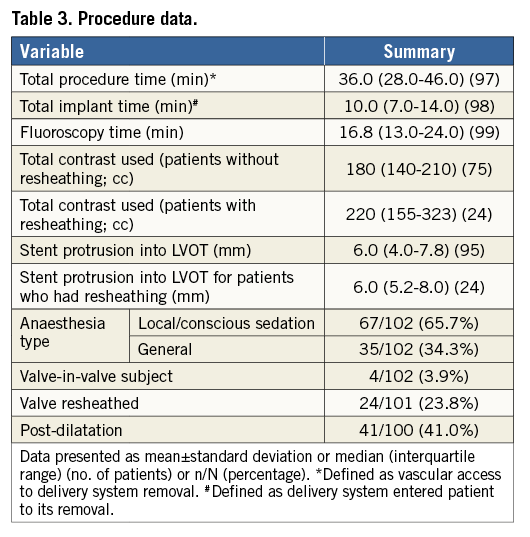
The overall device success (defined as successful vascular access, delivery and deployment of a single valve) was 92.2%. The valve was resheathed in 24 patients in total (23.8%) to optimise positioning. All resheathing procedures were successfully performed. Four patients required a second Portico valve (valve in valve). Three of these were deployed to correct haemodynamically significant PVL (due to the first valve being implanted too low) and one was to correct a migrated first valve (described above). The median depth of implant (stent protrusion into left ventricular outflow tract from annulus plane) was 6.0 mm. Post-dilatation following TAVI was performed in 41% of patients.
The median procedure time (obtaining vascular access to removal of delivery system) was 36.0 minutes, with a median fluoroscopy time of 16.8 minutes. The median total contrast used was 180 cc.
CLINICAL OUTCOMES AT 30 DAYS
The 30-day all-cause mortality rate was 2.9%. The causes of death were cardiovascular in two patients and hospital-acquired pneumonia in one patient. The cardiovascular deaths occurred due to procedure-related ventricular perforation and due to left main occlusion at day seven post implant – this patient required a second Portico 25 mm valve (valve in valve) as the first was inadvertently pulled and released supra-annular during removal of the delivery system, resulting in an increased skirt height overlying the left main coronary ostia, triggering reduced flow and occlusion.
Disabling stroke was observed in 2.9% of patients (Table 4): two occurred within 24 hours and the third occurred 24 days post procedure. Major vascular complications, overall, were low at 5.9%. Four of these patients had implantation of covered stents to treat either vascular site bleeding or dissection, one had suturing of an access site aneurysm and the other had post-procedure vascular site stenosis treated by percutaneous angioplasty. Life-threatening or disabling bleeding was observed in four patients (access site complications in two patients, pericardial effusion and bleeding following implantation of a permanent pacemaker in one and gastrointestinal bleeding in one patient). Stage 3 acute kidney injury post procedure occurred in two patients (2.0%). There were no episodes of annular rupture.
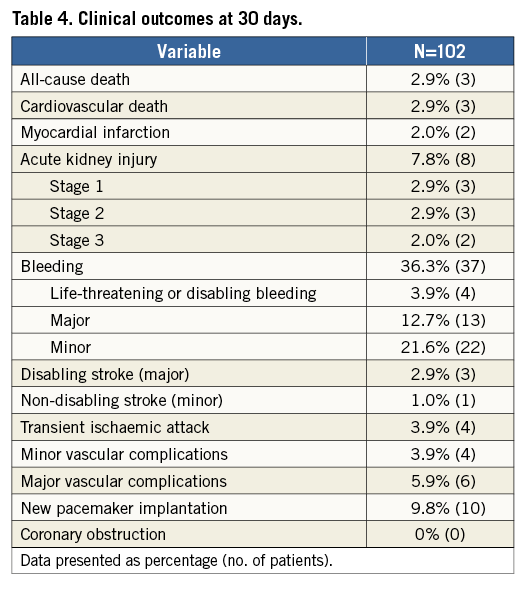
Most patients improved symptomatically following TAVI with the Portico system: 75.3% improved by at least one NYHA class and 25.9% improved by at least two NYHA classes (Figure 2). The six-minute walk distance increased from 174 m at baseline to 195.7 m at 30 days.
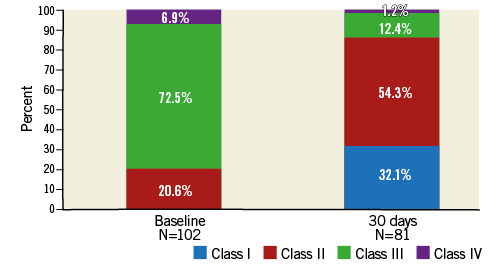
Figure 2. NYHA class at baseline and at 30 days post TAVI.
Permanent pacemaker implantation was required in 9.8% of patients post procedure. All received the pacemaker prior to discharge, with five patients requiring it immediately post implant (one with Mobitz II and four with complete heart block) and a further five requiring it pre-discharge (one with sinus arrest, one with sick sinus syndrome and three with complete heart block). The depth of implant was not statistically different between those who did and those who did not receive a permanent pacemaker after TAVI with the Portico valve.
ECHOCARDIOGRAPHIC OUTCOMES
Transcatheter aortic valve replacement with the Portico system effectively improved gradients across the aortic valve (45.3±13.8 mmHg to 8.9±3.8 mmHg; p<0001) and significantly increased valve area post procedure (0.6±0.2 cm2 to 1.7±0.4 cm2; p<0001) (Figure 3). These improvements were maintained at the 30-day echocardiographic follow-up. The majority of patients had PVL grade of trace/mild post TAVI at 30 days (96.2%), with moderate of 3.8% and none with severe (Figure 4).
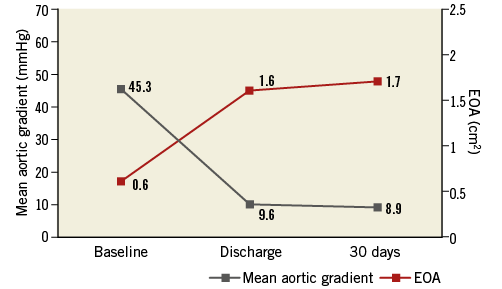
Figure 3. Echocardiographic haemodynamic measurements (mean±standard deviation).
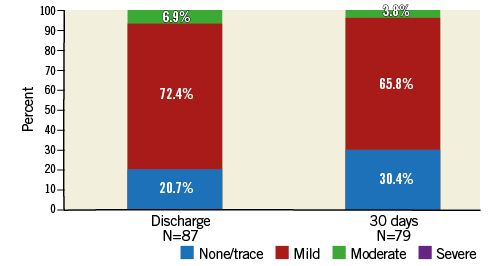
Figure 4. Paravalvular leak assessment post TAVI by echocardiography.
Discussion
This study demonstrates that the 23 and 25 mm Portico valves are safe and efficacious at treating high-risk symptomatic patients with severe aortic stenosis. The overall mortality and stroke rates at 30 days were low at 2.9% each. Although the study size is small, the 30-day mortality results appear favourable when compared with early-generation3,4,9,10 and newer-generation11-13 TAVI devices.
The rate of disabling stroke observed in this study was low (n=3) and appears similar to other newer-generation devices11-13. Repositioning attempts, repeated TAVI due to valve dislodgements and post-TAVI balloon dilatation have been associated with an increased risk of stroke14-17. We did not observe a correlation with the incidence of stroke in this study when resheathing, repositioning or post-dilatation was attempted with the Portico device.
Risk of vascular injury, annular rupture and coronary occlusion are potentially catastrophic complications of TAVI. Previous bioprosthetic AV, low-lying coronary ostia, female sex and use of balloon-expandable TAVI systems are risk factors for coronary occlusion18, whereas aggressive post-dilatation, balloon-expandable TAVI systems and severe annular calcification are associated with annular rupture19,20. The rate of major vascular complications observed was low at 5.9%. No annular rupture was observed in this study.
Resheathing was safely performed in all patients when attempted. A second valve was required in four patients. Although all centres were experienced TAVI centres using either Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) or Medtronic CoreValve (Medtronic, Minneapolis, MN, USA) TAVI systems, the need for a second valve was encountered during the early learning curve cases due to suboptimal positioning of the first valve (too low or too high). Upon careful review of these cases with the site investigators, it was felt the possible reasons for the valve in valve were complex patient anatomy and the early learning curve with the Portico TAVI system. Importantly, however, no difficulty with the delivery system was encountered during delivery, resheath or retrieval with any of the implants.
Self-expanding TAVI technologies are associated with increased requirements for permanent pacemaker implantation (11-40%) following TAVI4,21,22, when compared to balloon-expandable devices (3-8%)3,9,23. Better positioning and adherence to best practice have been shown to reduce the need for permanent pacemaker implantation in the ADVANCE II (13.3%) and EVOLUT R (11.7%) studies24,25. The repositioning feature of the Portico system may have contributed to the low rate of permanent pacing observed (9.8%). The larger cell areas (resulting in high porcine tissue:nitinol metal ratio) at the annular (skirt) contact zone may also have contributed to the lower rate observed due to the reduced trauma caused by the metal frame on the left bundle branch.
Moderate to severe PVL is associated with poor prognosis. The Portico valve was designed to mitigate the risk of PVL (trivial/mild: 96.2%). The larger cell design allows calcific protrusions at the annular zone to invaginate between the stent frames, allowing better apposition, resulting in better annular seal. The nitinol stent frame design further aids by exerting a consistent radial force within the annulus range for each valve size, thus conforming, yet adhering to the uneven landing zone (annulus).
Study limitations
The main limitations of this study are its relatively small sample size and the impact of the learning curve using a novel resheathable device. Due to the valve sizes used in this study, the results are limited to relatively small aortic annuli, corresponding to a majority of female patients in the study cohort. The study did not include, as per protocol, non-transfemoral approaches for TAVI.
Conclusions
The novel Portico TAVI system appears to be safe and effective at treating high-risk patients with symptomatic severe aortic stenosis. The system allows safe repositioning and optimisation of device position. Larger studies will help to validate the findings of this study.
| Impact on daily practice TAVI with the resheathable and repositionable Portico valve is safe, with low mortality (2.9%), low stroke (2.9%), low major vascular complication (5.9%) and low permanent pacemaker rates (9.8%). |
Acknowledgements
We thank Hongfei Guo, PhD (St. Jude Medical) for statistical analysis.
Funding
St. Jude Medical, St. Paul, MN, USA.
Conflict of interest statement
G. Manoharan has received consultancy fees and honoraria from Medtronic, St. Jude Medical and Boston Scientific. A. Linke has received speaker honoraria or served as a consultant for Medtronic, St. Jude Medical, Claret Medical Inc., Boston Scientific, Edwards Lifesciences, Symetis as well as Bard, and owns stock option from Claret Medical Inc. H. Möllmann has received consultancy fees and honoraria from Abbott, Boston Scientific, Medtronic, St. Jude Medical, and Symetis. S. Redwood is a proctor for Edwards Lifesciences and has received grant support from Edwards Lifesciences and Boston Scientific. The other authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Appendix. Study management and trial sites
Study sponsor: St. Jude Medical, St. Paul, MN, USA
Study coordinating investigators:
Dr Ganesh Manoharan, Royal Victoria Hospital, Belfast, UK
Prof. Thomas Walter, Kerckhoff-Klinik, Bad Nauheim, Germany
Study centres, site investigator/implanting clinician (total patients):
University of Leipzig, Heart Center, Germany: Dr David Holzhey; Prof. Axel Linke (37)
Royal Victoria Hospital, Belfast, UK: Dr Ganesh Manoharan; Dr Mark S. Spence (25)
Kerckhoff-Klinik GmbH, Bad Nauheim, Germany: Prof. Thomas Walther, Prof. Helge Moellmann (14)
St. Thomas’ Hospital, London, UK: Prof. Simon Redwood (9)
Glenfield Hospital, Leicester, UK: Dr Jan Kovac (9)
Asklepios Klinik, Hamburg, Germany: Dr Karl-Heinz Kuck, Dr Christian Frerker (6)
Echocardiography core laboratory:
Dr Sherif Nagueh, Methodist DeBakey Heart and Vascular Center, Houston, USA
Clinical events committee:
Cardiovascular Research Foundation, New York, USA
Chair: Roxana Mehran, Professor of Medicine, Mount Sinai School of Medicine, New York, USA

