Abstract
Transcatheter aortic valve implantation is increasingly being used to treat high-risk patients with symptomatic aortic valve disease. However, challenges still remain with current devices, both in terms of the procedure and the outcome. The St Jude Medical Portico transcatheter valve system is designed to mitigate some of these difficulties. We describe the device characteristics and how the device may impact on a TAVI procedure. An overview of the clinical experiences with the Portico valve system is also described.
Introduction
Trancatheter aortic valve implantation (TAVI) is being increasingly used to treat high-risk or inoperable patients with symptomatic aortic valve disease. Despite improvements with outcome, due to increasing operator experience, better patient selection and device iterations with current devices, challenges still remain. Ease of use, repositionability, retrievability, and reduced complications (paravalvular leak, vascular, stroke, permanent pacemaker implantation) are some of the desired improvements of the next generation devices. The Portico™ TAVI system (St Jude Medical, St Paul, MN, USA) is designed to be re-sheathable, repositionable, retrievable and potentially mitigate some of these challenges/difficulties. The device is currently available for investigational use only.
The Portico™ TAVI system
The Portico™ valve (Figure 1) is a trileaflet pericardial valve mounted inside a radiopaque self-expanding nitinol stent. It is designed to function at the annular level upon deployment. The valve leaflets are manufactured from bovine pericardial tissue and are sutured in a trileaflet configuration inside the stent frame. The cuff of the valve (in-flow portion or annular segment) is made from porcine pericardium and functions as a sealing zone. The large cell area design of the frame and the annular positioning of the functioning valve allow easier engagement of coronary ostia post implantation (Figure 2). Furthermore, the large cell area results in a high tissue to frame ratio at the annular/valve cuff segment, with a potential to minimise the risk of paravalvular leakage by allowing valve tissue to conform around calcific nodules at the annulus. The valve is processed with the Linx™ anti-calcification technology and is packaged, unloaded, in a preservative solution.
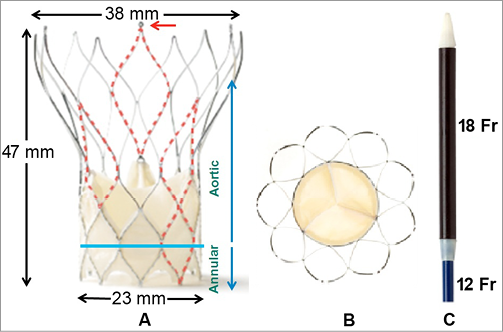
Figure 1. St Jude Medical Portico 23 mm transcatheter valve system. A) Large cell area in the aortic segment allows engagement of coronary ostia and in the annular segment allows tissue to conform around calcific protrusions, potentially minimising paravalvular leaks; red arrow showing 1 of 3 anchor tabs. B) Outflow portion demonstrating trileaflet configuration of the bovine pericardial valve. C) Distal end of delivery catheter showing radiopaque nose cone, loading segment of valve (18 Fr) and catheter (12 Fr).
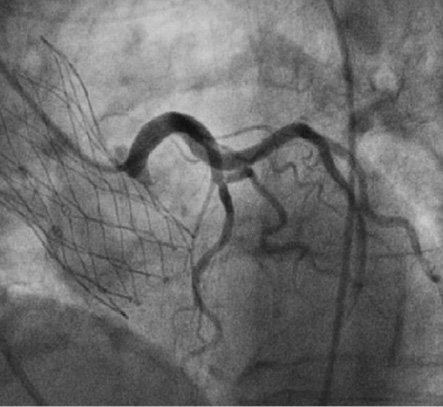
Figure 2. Coronary angiography of the left system post implant. The large cell area of the Portico system enables easy access to coronary ostias post procedure.
The delivery catheter is an over-the-wire, 0.035” compatible system. The outer diameter of the delivery system is 18 Fr at the distal end and 12 Fr at the proximal end (Figure 1). The distal tip is an atraumatic, radiopaque tip. After valve loading, a protective sheath covers and maintains the Portico™ valve in a collapsed profile. The handle is located on the proximal end of the delivery system and is used to load, deploy and re-sheath the valve. The system is designed to deliver the valve gradually, by the operator releasing the annular end of the valve implant first. The position of a partially deployed Portico™ valve can be evaluated and, if needed, it can be re-sheathed and redeployed, provided the stent has not been fully released from the delivery system (Figure 3, Moving image 1).
Patient selection: clinical and technical considerations
The Portico TAVI system is currently being evaluated in clinical studies, using the transfemoral route, to treat patients who have severe symptomatic aortic stenosis deemed high risk for surgical aortic valve replacement.
It is currently available as a 23 mm device, with larger valve sizes anticipated. The Portico™ 23 mm valve is designed to accommodate annulus diameters ranging from 19 mm to 21 mm. The loaded valve delivery system can be advanced into the patient via a standard 18 Fr femoral sheath, supported by a 0.035” stiff wire.
The minimum femoral and iliac access diameter is 6 mm, provided the vessel is not too calcified or tortuous. Key measurements (Table 1) for safe valve deployment include a sinus of Valsalva width of ≥27 mm with a height of ≥15 mm and an ascending aortic width (at 5 cm from annulus) ranging from 28 mm to 36 mm. Adherence to these parameters will reduce the serious risk of coronary occlusion post valve deployment; however, other features such as coronary ostia height and degree/orientation of cusp calcification should also be assessed, particularly in borderline cases.
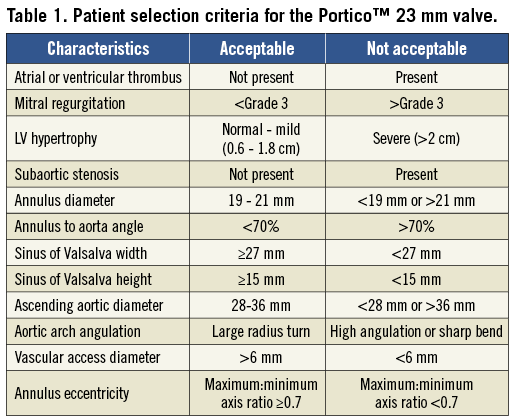
As the Portico™ valve is an annular functioning valve, severe eccentric annulus anatomy could potentially affect valve function. Following haemodynamic bench testing, a minimum to maximum annulus axis ratio measurement of ≥0.7 is therefore recommended to ensure correct valve function post deployment.
Procedural and deployment characteristics
The Portico system can be advanced through a standard 18 Fr femoral sheath. An 18 mm or 20 mm diameter valvotomy balloon (depending on annulus size) is used for predilatation. After positioning an Amplatz Super Stiff™ (Boston Scientific, Natick, MA, USA) or an Amplatz Extra-Stiff (Cook Medical, Bloomington, IN, USA) wire in the left ventricular cavity, the valve is advanced slowly up the descending aorta, across the arch and positioned at the annulus (Moving image 1). An aortogram is taken to ensure that the most distal segment of the valve is approximately 1 mm to 2 mm sub-annular and in alignment. The valve is gradually released, without rapid pacing, with angiography taken periodically to maintain position and depth (3 mm to 6 mm; Figure 3, Moving image 3). After full annular contact and 80% release, valve position and depth should be carefully assessed - there is no need to rush to release the valve as it is fully functioning at this juncture.
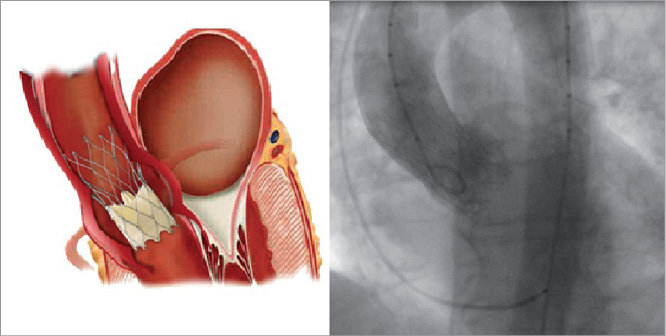
Figure 3. Optimal position of the Portico™ valve. Final post-procedure angiography demonstrates a competent valve with an implant depth of 3 mm. Optimal implant position is between 3 mm and 6 mm in depth.
If at this point, or at any point leading up to 80% release, the valve migrates out, then the valve can easily be re-sheathed (Figure 4, Moving image 2). The degree of re-sheathing that is required can be tailored depending on how much the valve has migrated: if the valve appears just marginally too high, then partial re-sheathing will allow repositioning to be carried out without the need to undergo the whole deployment process again; however, if the annulus requires re-crossing then full re-sheathing can be performed safely and the valve redeployed. If the valve is marginally too low, then gentle traction to the delivery system is recommended to move the valve higher before release. If optimum positioning is not achievable by this manoeuvre, then partial or full re-sheathing is recommended to reposition the valve.
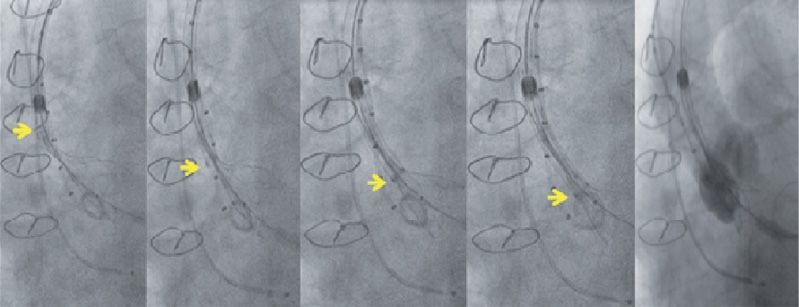
Figure 4. Re-sheathing of the Portico™ valve. Arrow marking the gradual re-sheathing of the valve at varying stages. Full re-sheath from 80% release takes approximately 15 seconds.
On full release, it is important to ensure all three valve anchor tabs attached to the delivery catheter are released prior to withdrawing the catheter. It is recommended that at least two differing views are taken to confirm this. The valve function is then assessed using standard methods and if post-dilatation is required due to an underdeployed valve, valvotomy with a maximum diameter balloon of 22 mm is recommended. It is vital that this is performed under rapid pacing at a rate adequate to reduce cardiac output significantly (usually between 180 and 220 beats per min). Failing to do this could result in the balloon being pushed out into the ascending aorta during maximum inflation, pulling the valve with it.
Overview of clinical data
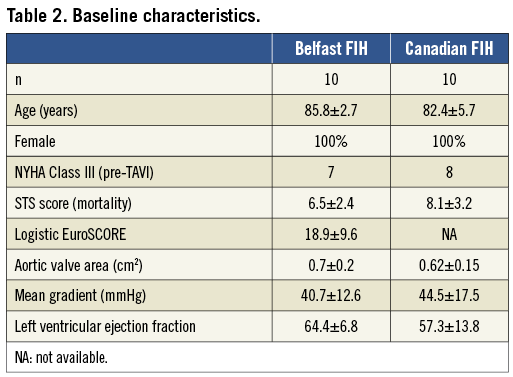
The 23 mm Portico™ valve has been evaluated in two feasibility and procedural outcome studies from Canada (St Paul’s Vancouver and Quebec Heart and Lung Institute, Quebec City, Canada)1 and Europe (Royal Victoria Hospital, Belfast, United Kingdom). Baseline characteristics are summarised in Table 2. Patients were exclusively females (n=20) due to the annular size range suitable for the Portico™ 23 mm valve.
The procedure was successfully completed in all patients, with valve deployment, removal of the delivery system, and percutaneous vascular closure. There was no reported death, major stroke or myocardial infarction at 30 days (Table 3). One patient had a minor vascular complication. Re-sheathing was required in six patients to optimise valve positioning and was completed safely and easily. A second valve (valve-in-valve) was required in one patient, with a calcified eccentric annulus and a small hypermobile calcific valvular nodule seven days post procedure due to intermittent severe regurgitation, resulting in complete resolution1. The cause for this is not clear but was felt to be due to a combination of a low positioned valve, eccentric annulus, eccentric calcification and paravalvular leak.
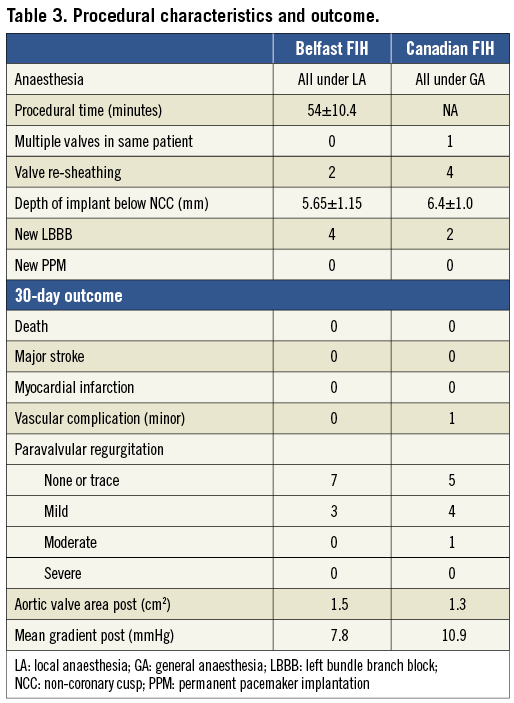
New left bundle branch block developed in six patients, with none requiring a permanent pacemaker. Significant improvements in valve haemodynamics observed post procedure were maintained at 30 days. Paravalvular regurgitation was trivial or less in 12 patients (60%), mild in seven (35%), and moderate in one (5%) (Table 3).
Conclusions
These encouraging early results would suggest that TAVI with the St. Jude Medical Portico™ 23 mm device is feasible and safe. The novel re-sheathable feature of the system can be easily and safely used to optimise valve position. Longer-term outcome data from this cohort of patients, the on-going European Portico 23 mm TAVI study, larger valve size studies (due later this year) and other access route studies would contribute to further evaluation of this new TAVI system.
Conflict of interest statement
G. Manoharan is principal investigator for the Portico FIH Study and proctor for the Portico TAVI programme. M. S. Spence is a co-investigator for the Portico FIH Study. J. Rodés-Cabau and J.G. Webb have no conflict of interest to declare.
Online data supplement
Moving image 1. The St Jude Medical Portico™ 23 mm valve, loaded on the delivery catheter is advanced via the femoral artery, across the arch and positioned just at the annulus. The valve is gradually released, without the need for rapid pacing. The position and depth of the valve can be checked periodically with contrast injection. At 80% deployment, final position should be confirmed before full release. The valve is functioning at this juncture.
Moving image 2. Re-sheathing of the St Jude Medical Portico™ 23 mm valve during TAVI in a patient is shown. Contrast angiography at intervals to check position is recommended during re-sheathing as full re-sheathing to reposition may not be required if the valve position is only marginally suboptimal.
Moving image 3. Final angiography post implant of a St Jude Medical Portico™ 23 mm valve is shown. The valve is competent, with both the right and left coronary systems filling well.

