Description - technical specifications
The heart leaflet technology (HLT; HLT Inc., Maple Grove, MN, USA) valve is a self-expanding design that is composed of four primary elements: i) a glutaraldehyde cross-linked tricuspid porcine pericardial tissue valve, ii) a superelastic nitinol wire frame that supports the valve structure, iii) a superelastic nitinol mesh, which supports the prosthetic valve and keeps the valve fixed within the native valve annulus, and iv) a braided polyester liner integrated within the support structure which is designed to prevent regurgitant flow around the valve (Figure 1).
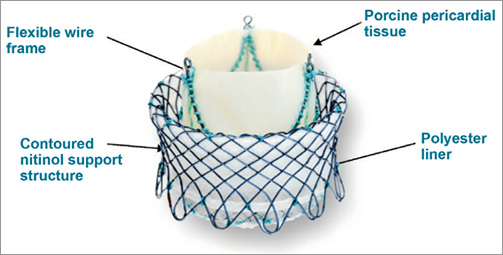
Figure 1. Deployed HLT valve in inverted mode.
DELIVERY SYSTEM DESCRIPTION
The delivery system comprises three primary components: i) the delivery catheter, ii) the delivery system handle, and iii) the HLT guidewire. The delivery catheter is 18 Fr (outer diameter [OD]) and is pre-curved on the distal end to accommodate the aortic arch. The delivery catheter houses a series of wires and connectors that attach to the valve at both the distal and proximal ends and support the valve during the implant procedure. The distal tip of the delivery catheter also utilises an atraumatic nose cone to facilitate passage through the vasculature and across the native aortic valve. Figure 2 shows (i) the distal end of the delivery catheter with the valve connected, and (ii) the distal end of the delivery catheter after the valve has been loaded.
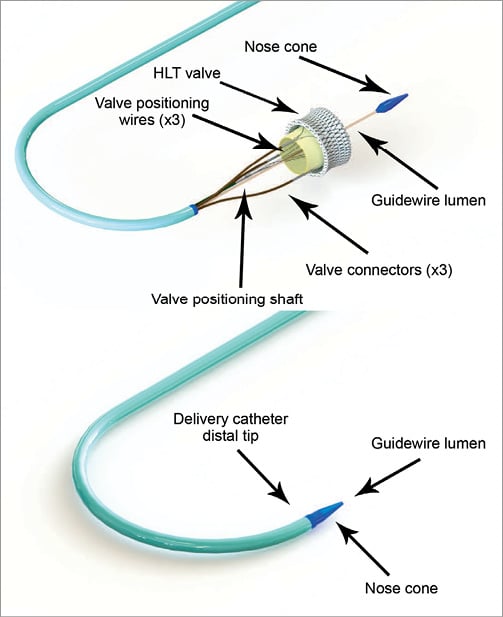
Figure 2. Delivery catheter distal tip.
The delivery system handle (Figure 3) has multiple components including: i) a valve deployment dial used to express, reposition or retrieve the valve, ii) a series of valve positioning components (collectively referred to as valve positioning mechanism) used to stabilise the valve as it is inverted within the native aortic annulus, iii) the valve connector release levers (×3) that control the valve connectors at the distal end of the catheter either to lock the valve connectors to the valve wire form during the valve loading process, or to release the valve from the valve connectors following a successful implant, iv) the safety release pin to secure the valve connector release levers during the valve implantation, and v) the valve retrieval release lever which is used to deactivate the valve deployment dial, allowing the valve to be pulled back into the delivery catheter, if valve retrieval becomes necessary.
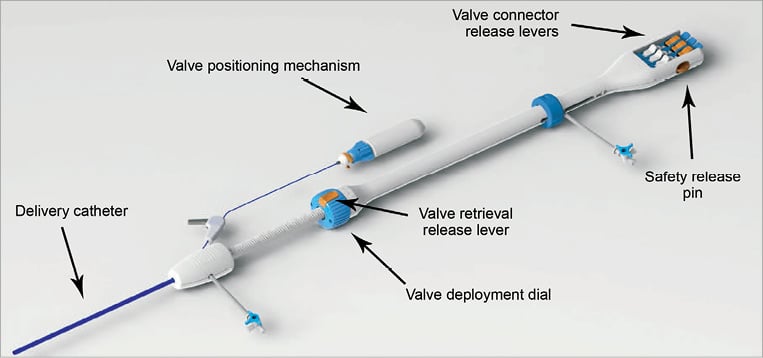
Figure 3. Delivery system handle.
The HLT guidewire is a standard 0.035” guidewire that has been extended to 350 cm to accommodate the length of the delivery catheter and delivery system handle.
Implant procedure
The HLT valve implant procedure includes many elements common to other percutaneous valve or structural heart repair technologies. Prior to use, the HLT valve is loaded into the delivery catheter. The device is delivered via the femoral artery and access can be obtained using conventional percutaneous puncture. Catheter guidance, valve positioning and delivery are achieved utilising standard fluoroscopic imaging techniques and planes. There are several specific procedural steps related to the HLT valve. After standard aortic valvuloplasty, the HLT delivery catheter is advanced, in over-the-wire fashion, across the native aortic valve into the left ventricular outflow tract. The HLT valve is then expressed from the delivery catheter and positioned within the native aortic valve. Once positioned, the HLT valve is then further expressed, while being controlled by the valve positioning mechanism, until the valve inverts upon itself to generate sufficient radial force to anchor the valve firmly. Following inversion, the remainder of the valve is then deployed from the catheter to initiate full valve function. Correct valve position and function are verified by TEE and fluoroscopic imaging. Importantly, the valve can be retrieved and the procedure re-initiated if the desired results are not achieved. If the results are satisfactory, the valve connectors are released, the delivery system and guidewire are removed and the procedure completed.
Clinical data
During the initial development stage an integral element of the device was a “back stop” which was thought to be essential for inversion of the valve (Figure 4). During the first-in-man trials performed at two sites in Germany in 2011 the back stop was responsible for serious ventricular injuries. A total of six patients were implanted with a 23 mm valve. Implantation was successful in five patients; however, there was ventricular perforation in one patient and chordal rupture in one patient. As a consequence, no further implantations were attempted and it was decided to redesign the entire valve. In five patients in whom the valve was deployed successfully, long-term (12 months) performance was good with only trace mitral regurgitation and no change of transvalvular gradient over time. Animal trials based on the new design without back stop have been initiated and have yielded promising results.
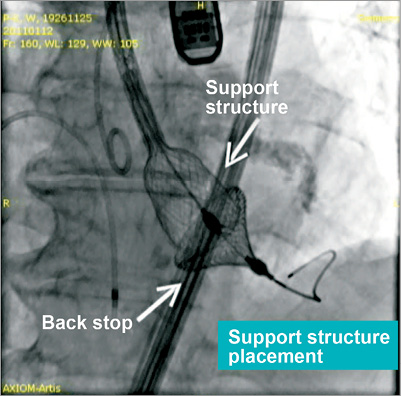
Figure 4. Initial valve design with “back stop”.
Conclusion
The HLT valve is based on a unique design using inversion of the nitinol stent for creating sufficient radial force to anchor the device in the aortic annulus. For passage through the pelvic vessels the device assumes an elongated configuration with the support structure and biologic valve travelling in sequence. Thus, very small cross-sections of the deployment assembly are feasible. Elimination of the back stop has improved handling and safety characteristics considerably; the control wires reliably cause the support structure to invert on itself, once it has reached the correct position in the aortic annulus. The valve can be fully deployed and removed as long as it is still attached to the valve connectors. One-year data have shown good durability of the valve design without changes in valve area or mean gradient.
Conflict of interest statement
R. Schroeder is an employee of HLT. A. Linke and G. Schuler have no conflicts of interest to declare.

