Abstract
Transcatheter aortic valve implantation (TAVI) has been adopted worldwide as a prominent therapeutic alternative for patients with high-risk severe symptomatic aortic stenosis. Despite its more widespread adoption as a treatment option and the increasing experience of the centres, TAVI is still associated with complications. Therefore, improvements in transcatheter heart valve (THV) technology, such as optimisation of device function with superior deliverability, and valve repositioning, are desirable. Accordingly, this article describes the new self-expandable Edwards CENTERA THV, made of nitinol and treated bovine pericardial tissue, and delivered by a motorised low-profile delivery system which potentially permits accurate valve placement and valve repositioning by a single operator. First-in-human clinical experience with the CENTERA THV has been promising but larger series with longer follow-up are warranted to confirm these initial positive results.
Introduction
Transcatheter aortic valve implantation has been adopted worldwide as a prominent therapeutic option for patients with high-risk severe symptomatic aortic stenosis1. Nowadays, there are two different transcatheter heart valves (THV) commercially available that have been widely used, with extensive data on feasibility, safety and clinical outcomes1. However, newer generations and types of THV are emerging, possessing several potential advantages that might improve the limitations of current systems. These newer generation valves have been developed for improved valve function, with higher effective orifice areas and lower rates of paravalvular regurgitation, with superior deliverability and lower profile, easier positioning and repositioning capabilities, and lastly with better durability as compared to surgical valves. This article describes the new self-expandable Edwards CENTERA THV system (Edwards Lifesciences, Irvine, CA, USA) and its future perspectives.
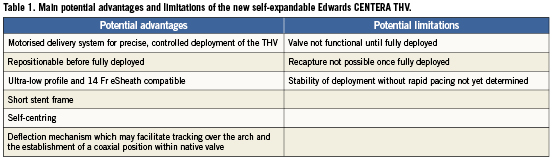
Characteristics of the Edwards CENTERA THV system
The Edwards CENTERA THV is an ultra-low-profile, self-expandable valve that consists of three treated bovine pericardial tissue leaflets attached to a nitinol frame (Figure 1). Currently, the valve is available in 23 mm and 26 mm sizes (where 23 mm and 26 mm are the nominal diameters at the waist of the valve, respectively). An additional larger valve size of 29 mm is also anticipated. In contrast to other self-expandable valves1, the stent frame is shorter (17.5 mm and 20 mm for 23 mm and 26 mm valves, respectively) and therefore does not have a flared distal section that extends into the ascending aorta. The stent frame’s shape potentially facilitates centring and seating of the valve within the annulus, and it may also help to improve paravalvular sealing with minimal protrusion of the valve frame into the left ventricle.
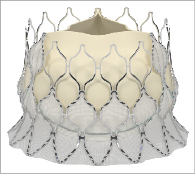
Figure 1. The CENTERA transcatheter heart valve consists of a nitinol self-expandable stent, treated bovine pericardial leaflets, and polyethylene terephthalate (PET) fabric.
The delivery system consists of a delivery catheter and a detachable, battery-powered motorised handle, which can be delivered by the transfemoral or subclavian approaches. It is motorised for stable deployment and to allow for single-operator use. The capability to re-sheath and reposition in situ prior to complete valve deployment is an expected feature and may reduce the risk of valve malposition or embolisation. The initial delivery system was compatible with an 18 Fr sheath (outer diameter) (Figure 2). The current CENTERA THV is under clinical testing with a new delivery system and is expected to be compatible with a 14 Fr eSheath (Edwards Lifesciences, Irvine, CA, USA). The new version of the system has a shorter valve section, catheter shaft deflector, and a tapered distal end, in order to improve tracking and optimise co-axiality (Figure 3). The eSheath system has the dynamic expansion mechanism (DEM) that allows for transient sheath expansion during valve delivery (Figure 4). Immediately after the valve passes through the sheath, the DEM allows the sheath to return to a low-profile diameter. This reduces the time the access vessel is expanded, thereby potentially minimising the risk of vascular trauma.
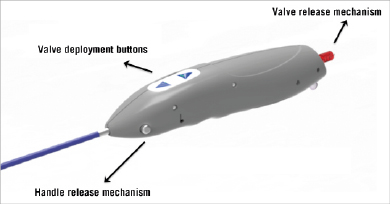
Figure 2. Initial version of the motorised delivery system with buttons that control valve loading and deployment, and with handle release button and valve release mechanism, which permits the final release of the device.
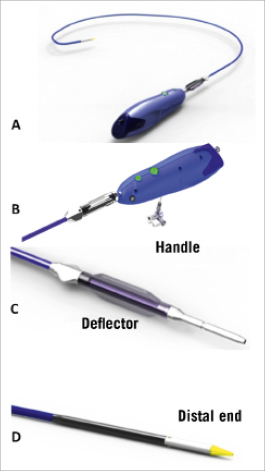
Figure 3. Newer version of the CENTERA motorised delivery system (A) with improved tracking and co-axiality: B) handle; C) deflector; D) tapered nose cone
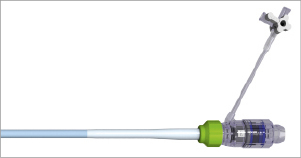
Figure 4. eSheath.
The potential advantages and limitations of the CENTERA THV system are highlighted in Table 1.
Valve implantation process (transarterial approach)
The valve implantation process commences with the advancement of the delivery catheter into the left ventricle (Figure 5A). The ventricular edge of the valve is slightly flared and is positioned around 2-4 mm below the annulus, offering minimal protrusion into the left ventricle (Figure 5B). This initial deployment of the valve is achieved by the motorised handle, which has two buttons. These buttons control the delivery cylinder, which permits the retraction of the cylinder to deploy the valve or advancement of the cylinder to load the valve, allowing a more precise and controlled deployment. Once the operator is satisfied with the positioning, full deployment is achieved by complete retraction of the delivery cylinder (Figure 5C). During deployment, the position of the THV can be assessed and, if not satisfactory, THV recapture can be accomplished by pressing the loading button of the delivery system. Following recapture, the THV can be redeployed or removed. The safety of repeating the re-sheathing process more than once is currently unknown, pending completion of preclinical testing. Moreover, the valve can only be recaptured prior to ~70% of its deployment and not after full deployment. Final adjustments of the valve position are possible during this deployment stage. When the operator is satisfied with the final position, the valve is fully deployed by pressing the deployment button (Figure 5C). It is important to be aware that this THV obstructs left ventricular outflow at approximately 50% of its deployment: hence, rapid ventricular pacing was recommended in the initial experience during the final deployment stage (between ~50% and full deployment, usually lasting 3-5 seconds). Of note, in the future, deployment of the prosthesis without rapid pacing may be possible. Detachment of the valve from the delivery system is achieved by utilising the valve release mechanism located at the bottom of the handle (Figure 3).
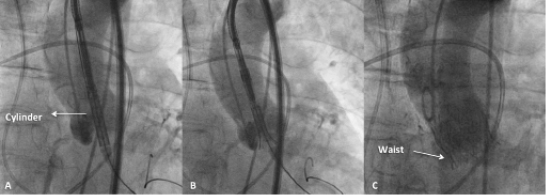
Figure 5. Delivery and deployment of the CENTERA THV system (courtesy of Professor Karl-Heinz Kuck and Dr Ulrich Schäfer, Asklepios Klinik St. Georg, Hamburg, Germany). A) CENTERA system crossing native annulus. B) Initial flare and positioning of CENTERA system in preparation for deployment. C) Final deployment and position of the CENTERA valve.
First-in-human experience
The first-in-human experience with the 26 mm CENTERA valve took place in two centres, St. Paul’s Hospital in Vancouver, BC, Canada, and the Asklepios Klinik St. Georg, Hamburg, Germany, between May and December of 20102. In this initial experience which included ten patients, the CENTERA valves were successfully implanted without major procedural complications.
Following the modification of the delivery system (Figure 3), another five patients were evaluated in Canada (St. Paul´s Hospital, Vancouver, BC, Canada), between February and March 2012. Valve implantation was successful in all cases with no major procedural complications.
In summary, the early first-in-human experience showed that the implantation of the new CENTERA THV for the treatment of patients with severe symptomatic aortic stenosis is feasible. Nonetheless, these initial results will have to be confirmed by larger studies.
Future perspectives
Further enhancements to the CENTERA THV system have been made recently, in addition to those described above, and clinical experience with this newer system has commenced in June 2012 at the Asklepios Klinik St. Georg, Hamburg, Germany. To date, ten patients have been treated with this new system; however, clinical outcome data are pending. A European CE mark clinical approval trial is also scheduled to begin with this new system in late 2012.
Conclusions
The self-expandable CENTERA is one of the latest THV in clinical development from Edwards Lifesciences. It incorporates several novel designs into its low-profile motorised delivery system, enabling stable deployment and the possibility to recapture and redeploy, all performed by a single operator. First-in-human clinical experience with the CENTERA THV is promising, but larger series with longer follow-up are warranted to confirm these initial positive results and to ensure valve performance and durability. Likewise, a randomised trial will be needed to evaluate if these potential advantages of the CENTERA THV will translate into meaningful clinical outcomes as compared to the currently available THVs.
Conflict of interest statement
K. Kuck, J.G.Webb and J. Rodés-Cabau are consultants for Edwards Lifesciences. The other authors have no conflicts of interest to declare.

