Abstract
The transcatheter aortic valve prosthesis ACURATE TA™ (Symetis SA, Ecublens, Switzerland) has been specifically designed for transapical access with a future transfemoral version under development. The concept relies on a self-expandable nitinol stent housing a regular porcine bioprosthesis. A unique two-step implantation technique facilitates intuitive positioning. Two pre-approval multicentre trials have demonstrated promising results especially as regards a low rate of paravalvular leaks. The device obtained CE mark at the end of 2011 and is currently commercially available in Europe. This manuscript focuses on the technical details of this new transapical TAVI device and its specific implantation technique.
Introduction
Over the last few years transcatheter aortic valve implantation (TAVI) has evolved towards a routine procedure to treat elderly high-risk patients suffering from severe symptomatic aortic valve stenosis. Today, several options as regards the access are available. Prostheses can either be implanted using a retrograde endovascular approach (transfemoral TF, trans-subclavian TS, transaortic TAo) or via the antegrade transapical approach (TA).
In Europe two different TAVI devices became commercially available in 2008 (CoreValve®; Medtronic, Inc., Minneapolis, MN, USA; and Edwards SAPIEN™; Edwards Lifesciences, Irvine, CA, USA) and several thousand TAVI procedures have been performed to date using these devices. After an initial pioneering phase, current European results indicate a 30-day mortality rate of 6-13% mainly depending on a patient’s baseline risk profile1,2. At present, TAVI (both TF and TA) can be considered as a routine procedure at many high-volume European centres with ever increasing annual numbers of procedures being performed.
However, there are several issues associated with the TAVI technique which have not yet been solved. The procedure requires a significant learning curve. This has been shown to be true for both long-approved devices (CoreValve® and Edwards SAPIEN™) in particular with regard to precise valve positioning3,4. In addition, rates of AV blocks requiring permanent pacemaker implantation are still high, especially after CoreValve® implantation5. Most importantly, residual paravalvular leaks >1+ have recently been identified as having a significant impact on survival6-8. Thus, it is obvious that further refinements of existing devices and the development of new TAVI device concepts are required. In addition, the Edwards SAPIEN™ prosthesis was until recently the only device that facilitated an antegrade transapical implantation, indicating the general need for more therapeutic (TA device) options.
THE ACURATE TA™ DEVICE
The ACURATE TA™ device (Symetis SA, Ecublens, Switzerland) has been specifically developed for the antegrade transapical approach focusing on an intuitive implantation technique. A similar device for retrograde TF implantation is currently under development.
STENT DESIGN
The stent frame is a self-expanding nitinol frame (Figure 1) that was designed to facilitate a simple single-operator two-step implantation technique. Three key features should be mentioned. At the distal edge of the stent body three “stabilisation arches” are mounted to prevent tilting of the prosthesis during final deployment. The so-called “upper crown” is formed by the most distal part of the stent body and is meant to embrace the native calcified leaflets as well as to provide “tactile feedback” during final deployment. The stent commissures are well visible under fluoroscopy with a “circular” radiopaque appearance which facilitates anatomical rotation of the prosthesis (commissural alignment).

Figure 1. Symetis ACURATE TA™ stent.
TISSUE VALVE
Within the nitinol stent a biological tissue valve is mounted (Figure 2). The valve is similar to regular surgical porcine tissue valves with regard to leaflet thickness as the TA design does not require excessive “crimping” of the leaflets. Three different sizes are available allowing for treatment of patients presenting with an annulus diameter (AD) ranging from 20 mm up to 27 mm . To prevent confusion with sizing strategies of other established devices, valves are labelled small (S: AD 20-23 mm ), medium (M: AD 23-25 mm ) and large (L: AD 25-27 mm ).
LEAK SEALING AND LVOT CONFIGURATION
To seal paravalvular leaks, a PET skirt is mounted at the proximal (intra-annular) part of the stent body (Figure 2). In addition, the specific implantation technique is meant to compress native calcified leaflet tissue in between the “upper crown” and the annular level. To prevent compression of conduction tissue the proximal part of the stent has been designed with minimal protrusion into the LVOT.
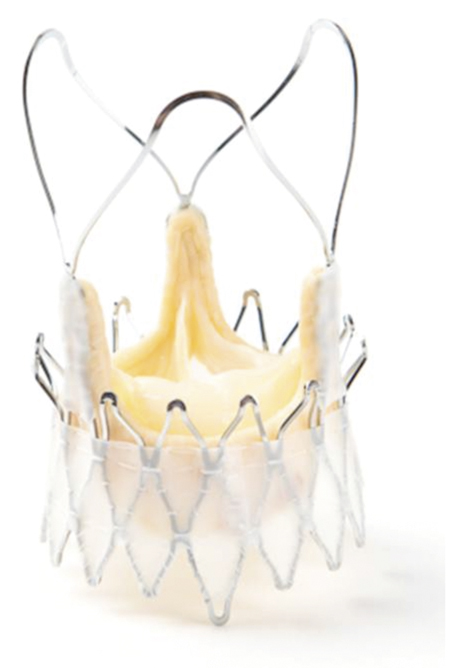
Figure 2. Symetis ACURATE TA™ bioprosthesis.
DELIVERY SYSTEM
The delivery catheter (Figure 3) is based on a sheathless concept similar in size to a 28 Fr sheath system. The prosthesis is “loaded” onto the delivery device and fully covered by the valve housing, resulting in an isodiametric shaft to ease insertion into the ventricle. Three markers at the distal handle can be used to ease commissural alignment and to help the operator to maintain proper anatomical rotation during the implantation process. Valve deployment is facilitated using a simple rotational knob suitable for a single-operator technique and a “safety pin” preventing accidental full release. Until final release the system allows for re-sheathing and repositioning.
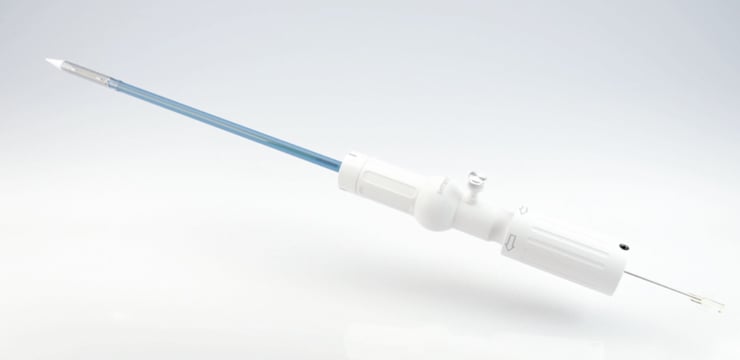
Figure 3. Symetis ACURATE TA™ transapical delivery system.
Implantation technique of the ACURATE TA™
TA ACCESS
Access to the left ventricular apex is established surgically similar to the technique used for Edwards SAPIEN™ implantation. This has been described in detail previously9. Briefly, an anterolateral minithoracotomy using a 5 cm skin incision is performed followed by exposure of the left ventricular apex by pericardial stay sutures. After identification of the optimal access site (slightly cranial of the true apex, safely lateral of the LAD) two pledged supported purse strings are placed to secure the access site. After puncturing, a soft J-tip wire is advanced antegradely through the stenotic aortic valve and manipulated across the aortic arch with the help of a Judkins right catheter which is then used in order to exchange for a super stiff guidewire placed in the descending aorta.
OPTIMAL C-ARM ANGULATION
For implantation of the ACURATE TA™ device the optimal fluoroscopic view is to obtain a C-arm angulation perpendicular to the annular plane with the native valve commissure between the left and right aortic cusp in the middle of the fluoroscopic projection. C-arm angulation can be established either by using enhanced imaging like the DynaCT (Siemens AG, Erlangen, Germany) (Figure 4B)10 or by repeat root angiography and adjustments of the angulation (Figure 4A). Usually, an angulation around LAO 30° CRAN 15° will be a good first guess.
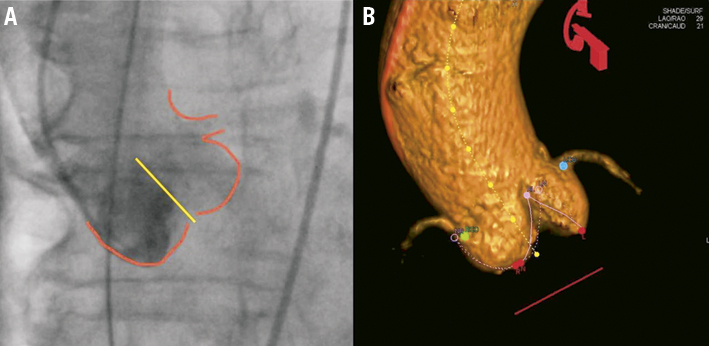
Figure 4. Optimal C-arm angulation. A) Conventional root angiography; B) DynaCT
BALLOON VALVULOPLASTY
Prior to valve implantation, a balloon valvuloplasty is strongly recommended. Initially, balloon valvuloplasty was performed with relatively small balloons. However, as radial forces are less than with a balloon-expandable system, we now advocate valvuloplasty with a balloon fully matching the annulus diameter in order to avoid post-dilatation after the valve has been deployed.
Anatomical, commissural alignment
After balloon valvuloplasty has been performed, the delivery device housing the valve is inserted into the ventricle over the wire in a blunt manner and advanced under fluoroscopic guidance until the radiopaque marker matches the annular plane (aligns with the proximal edge of the pigtail catheter in the non-coronary cusp). In case of a very short ascending aorta, the valve housing can be slightly unsheathed, which will significantly increase the flexibility of the system and will subsequently ease further advancement of the delivery system tip into the aortic arch.
Although not mandatory, anatomical commissural alignment can now be performed. One of the radiopaque “circles” indicating a device commissure is aligned with the middle of the fluoroscopic annulus projection (aligning to the known position of the left-right native commissure, Figure 5). A further manoeuvre is required to rule out the possibility that the targeted device commissure (radiopaque circle) is in the “back” instead of “in front” which cannot be discriminated given the limitations of a monoplane fluoroscopic system. Therefore, a clockwise rotation (rotational crosscheck) of the device is performed which should lead to a movement of the targeted device commissure (radiopaque circle) in the direction of the left main stem (Moving image 1). As has already been mentioned, anatomical commissural alignment is not mandatory but can usually be performed safely within a few seconds: it will mimic a physiological orientation of leaflets as well as avoid device commissures in front of coronary ostia.
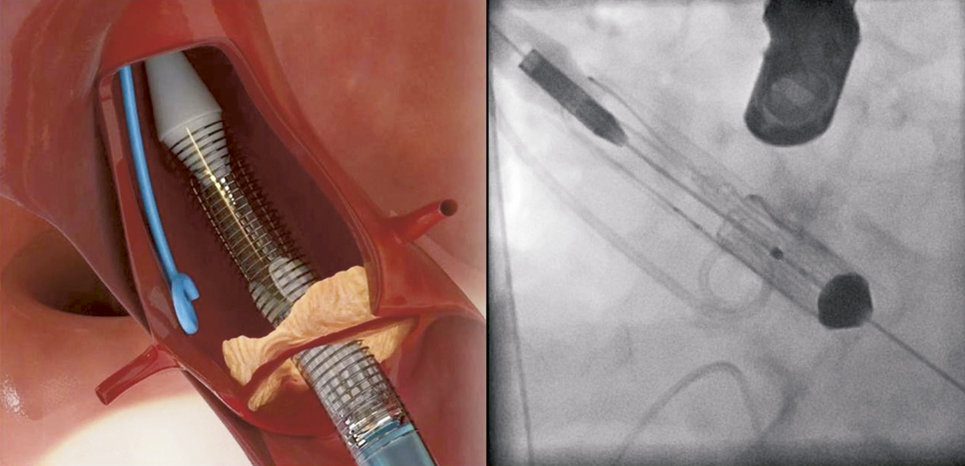
Figure 5. Initial positioning of the ACURATE TA™. The two radiopaque markers should bisect the annular plane indicated by the pigtail catheter.
IMPLANTATION: STEP 1
In the first step, the device is partially unsheathed at the distal part by rotating the delivery knob at the handle until stopped by the safety pin. Step 1 will release the “stabilisation arches” as well as the “upper crown” (Figure 6) and does not require rapid pacing. It is important to note that at this stage of the implantation sequence the “upper crown” is still above (distal) of the native calcified leaflets.
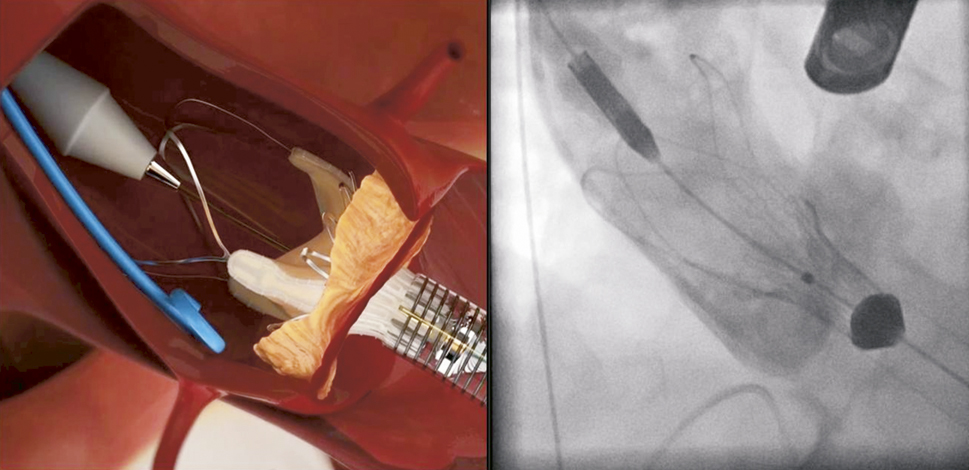
Figure 6. ACURATE TA™ implantation: step 1. The prosthesis is partially un-sheathed releasing the stabilisation arms and the upper crown of the stent body.
IMPLANTATION: STEP 2
After removal of the safety pin the valve is ready for final deployment. We advocate implantation under rapid ventricular pacing (RVP) which in our opinion eases precise placement and enhances visualisation. However, RVP is not mandatory and valve implantation can be performed on the beating heart which might be advantageous in patients with poor left ventricular function.
The following implantation technique is used in the majority of cases. After the ventilation is disconnected RVP is started and “no output” is confirmed followed by a root angiography over the pigtail catheter in the non-coronary cusp. The contrast injection during RVP will result in a fully contrasted aortic root providing optimal visualisation during the following valve deployment. Now the operator applies slight tension onto the delivery system (gentle pulling) which will bring the “upper crown” into contact with the calcified leaflets. This will be noticed by the operator by way of “tactile feedback”. The upper crown compresses the calcified tissue while at the same time safely embracing the leaflets. This should reduce the risk of coronary impingement. While maintaining the tension the prosthesis is now fully released by further turning of the delivery knob (Figure 7, Moving image 2). In the rare case that the prosthesis is accidentally pulled into the left ventricle (due to too much force, minimal calcifications or borderline large annulus) the delivery system allows for re-sheathing and subsequent repositioning until full deployment. Re-sheathing is performed by simply turning the delivery knob in the reverse direction. Once the prosthesis is deployed (Figure 8), RVP is stopped and the delivery sheath is retrieved, followed by snaring of the purse-string sutures. Valve function and position is then assessed by TEE and angiography (Figure 9, Moving image 3).
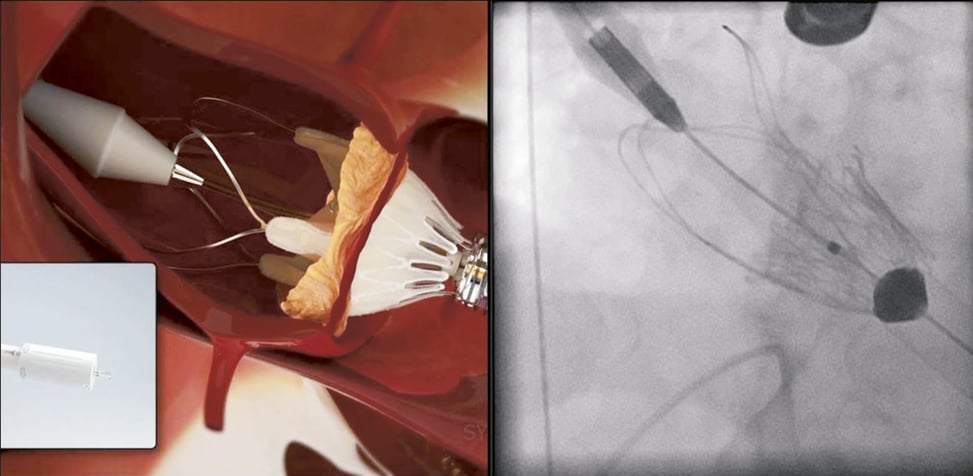
Figure 7. ACURATE TA™ implantation: step 2. During full deployment of the prosthesis the operator should gently pull on the delivery system to embrace the native calcified leaflets and compress the tissue by the upper crown. The system will provide solid tactile feedback during this manoeuvre.
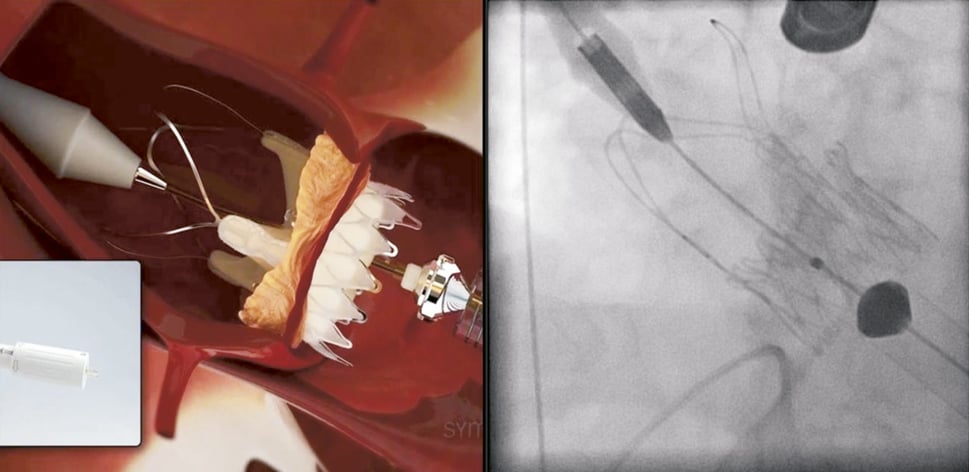
Figure 8. ACURATE TA™ implantation: full deployment. The stent body is fully deployed and self-detaches from the delivery system. Afterwards the delivery system is retrieved (no closing required).
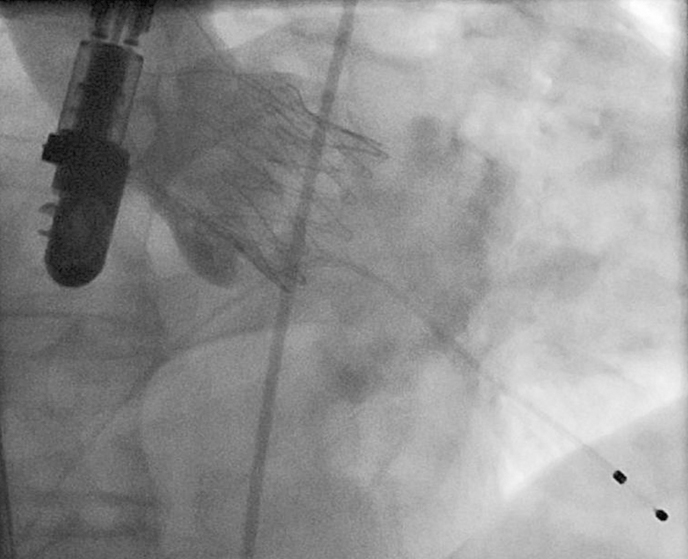
Figure 9. ACURATE TA™ implantation. Angiographic result demonstrating an intra-annular and fully subcoronary position of the stent body without any relevant paravalvular leak.
ACURATE TA™: clinical results
In an initial FIM trial 40 patients have been treated. Mean age was 83.2±4.0 years, mean logistic EuroSCORE I 21.2±10.8% and mean STS score 9.0±4.7%. According to VARC criteria11 device success rate (92.5%) and the combined 30-day safety endpoint were comparable to data from long-established devices12. Six-month follow-up data have been published recently13 and, as expected, demonstrate a significant clinical improvement with mean transvalvular gradients of 11 mmHg (mean EOA 1.5±0.4 cm2). The survival rate at six months was 82.5%, and 7.5% of patients required a new pacemaker implantation. Both rates compare favourably to current data after implantation of other established devices. The rate of residual paravalvular leaks looks very promising with only one patient demonstrating a 2+ leak and all others with either none/trace or mild regurgitation13.
The initial trial was followed by a second pre-approval trial (PILOT) which included another 50 high-risk elderly patients. The detailed results will be published soon. Again, the rate of paravalvular leaks was very promising, indicating that this might turn out to be device-specific. This would be a great achievement, as residual leaks >1+ have been proven to be associated with worse outcome.
Based on the dataset of these two multicentre trials (six German sites) the ACURATE TA™ device obtained CE-mark approval in September 2011. Since then, more than 150 commercial implants have been performed within a post-market registry (SAVI) demonstrating promising results especially with regard to paravalvular leaks.
Discussion
Although TAVI in general can be considered as a huge success story in Europe, several issues associated with the technique are still unsolved and are of concern. These problems are of particular importance when discussing the broadening of TAVI indication to younger patients or patient groups with lower risk profiles, given the excellent outcomes after surgical (minimally invasive) aortic valve replacement even in octogenarians14.
Both early TAVI systems underwent several technical refinements easing the procedures. Regarding the TF technique, the development of new device iterations which now allow valve delivery through lower-profile access sheaths can be considered as a major achievement. Similarly, for the TA technique a new Edwards SAPIEN™ delivery system has been introduced recently (Ascendra2™) in order to simplify the procedure further. To address the current shortcomings of TAVI, further refinements of established concepts as well as development of new TAVI devices are required. In addition, more TAVI devices on the market will result in some competition which is likely to stimulate innovation further. Hence, the development of new TAVI devices in general will result in more therapeutic options for our patients. The next step would be to identify which device concept through which access site is best suited for the individual patient.
Due to the fact that most centres follow a “TF first” strategy, patients who undergo a transapical procedure are usually at higher risk as indicated by risk scores or just simply by the fact that they carry a much higher calcium burden. Hence, direct comparison of outcomes, i.e., TF versus TA, as reported in the current literature, is not scientifically valid. In contrast, data from the Canadian multicentre SAPIEN™ experience15 as well as our personal experience indicate that TA-AVI outcomes compare very well to TF-AVI if patients have similar baseline risk profiles.
Bearing this in mind, the development of a new TAVI device specifically designed for the transapical approach makes perfect sense, as the transapical access offers several advantages especially for early generations of these devices: sheath diameters are not the major driving factor in contrast to the TF technique. This allowed the incorporation of a “regular surgical” porcine valve within the ACURATE TA™ device not reduced in leaflet thickness and without the necessity for tight crimping. Both factors might have an impact on durability.
Due to the short distance over the wire, most operators will agree that TA devices are usually easier to control. As the ACURATE TA™ device provides clear radiopaque markers of the device commissures, anatomical rotation (commissural alignment to the native valve) can be easily obtained. Whether this concept of commissural alignment provides any clinical benefit cannot be assessed at present. However, we believe that mimicking physiological leaflet orientation and avoiding commissural posts in front of coronary ostia might be beneficial with regard to flow patterns, risk of coronary impingement and ease of later coronary interventional procedures.
TAVI procedures are associated with a significant learning curve, something which has been scientifically proven for both early devices3,4. In addition to patient selection and screening, establishment of imaging technologies as well as post-procedural care, most operators will initially struggle with precise positioning of the prosthesis itself. The ACURATE TA™ concept might offer a relatively steep learning curve facilitating an intuitive positioning with tactile feedback and a concept that will theoretically result in a kind of “pre-set” valve position less dependent on the experience of the operator.
Recently, residual paravalvular leaks >1+ have been identified as an independent risk factor for mortality in several series6-8. Results observed with this new transapical device are very promising as regards the rate of leaks. Two factors might explain this observation. Firstly, the PET skirt at the proximal part of the stent might aid sealing against paravalvular leaks. Secondly, the specific implantation technique might also play a key role. Typically the “upper crown” of the ACURATE TA™ prosthesis is released above (distally) the native calcified leaflets during step 1 of the deployment. Then, during step 2 the “upper crown” is pulled down into the aortic annulus, thereby embracing and compressing the calcified tissue. This mechanism might further explain the low rate of relevant paravalvular leaks.
In contrast to the very promising features with regard to paravalvular leaks and ease of implantation, this new device also has some limitations. Due to the stent design together with the “stabilisation arches” the device is most likely less suited for valve-in-valve procedures. In addition, the nitinol concept offers lower radial forces than the balloon-expandable Edwards SAPIEN™ prosthesis. Thus, valvuloplasty should be performed quite aggressively to avoid post-dilatation which in our opinion always carries the risk of annular perforation independent of the type of device in place. Although re-sheathing and repositioning is feasible until final release, no retrieval is feasible after full deployment. Lastly, the current version of the device is still relatively large in diameter, which might become a limitation when transapical closure systems are used to facilitate a percutaneous TA procedure in the near future.
Based on the experience gained with the ACURATE TA™, a transfemoral version has been developed recently which is very similar to the TA device. This ACURATE TF™ prosthesis has entered a multicentre FIM trial recently with very promising early results.
Conclusion
The ACURATE TA™ device has been specifically designed for the transapical access. Key features of the device are a relatively simple unique implantation technique providing tactile feedback as well as a regular porcine valve not reduced in leaflet thickness. Two pre-approval multicentre trials have demonstrated promising results especially with regard to the rate of paravalvular leaks. The device obtained CE-mark approval at the end of 2011 and is currently implanted commercially with data collected within a post-market registry. A transfemoral version of the device has undergone first clinical trials successfully very recently.
Conflict of interest statement
J. Kempfert and H. Möllmann have received speaker honorarium from Symetis. T. Walther has no conflict of interest to declare.
Online data supplement
Moving image 1. Orientation of the Acurate™ prosthesis into an anatomical rotation by visualising one commissure and then by performing slight clockwise rotation. As shown in this clip the commissure moves towards the left main stem. Thus, it is clear that this is at the anterior circumference of the aortic root matching the native commissure between left and right coronary cusp.
Moving image 2. Full implantation of the Acurate™ prosthesis (step 2). The upper crow is open (step 1), and the valve is pulled slightly into the native calcified leaflets (tactile feedback) under fluoroscopic control, then the valve is fully unsheathed.
Moving image 3. Final angiogram showing the valve in perfect intraannular position with good haemodynamic function and patent coronary arteries.

