Abstract
The JenaValve™ is a next-generation TAVI device which consists of a well-proven porcine root valve mounted on a low-profile nitinol stent. Feeler guided positioning and clip fixation on the diseased leaflets allow for anatomically correct implantation of the device without rapid pacing. Safety and efficacy of transapical aortic valve implantation using the JenaValve™ were evaluated in a multicentre prospective study that showed good short and midterm results. The valve was CE-mark released in Europe in September 2011. A post-market registry ensures on-going and prospective data collection in “real-world” patients. The transfemoral JenaValve™ delivery system will be evaluated in a first-in-man study in the near future.
Introduction
With more than 50,000 implantations worldwide, transcatheter aortic valve implantation (TAVI) has become a viable treatment option for patients with severe aortic valve stenosis at high risk for surgical aortic valve replacement (AVR)1-3. The randomised controlled US PARTNER (Placement of AoRTic TraNscathetER Valve) trial revealed a significant reduction in mortality for TAVI using the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) in comparison to optimal medical treatment with or without balloon valvuloplasty (BAV) in inoperable patients4. In addition, multiple European registries have confirmed high procedural success rates and reliable clinical improvement for patients treated with either the balloon-expandable Edwards SAPIEN valve or the self-expanding Medtronic CoreValve™ (Medtronic, Inc., St. Paul, MN, USA)5,6. Despite these encouraging results some procedure-related problems remain to be solved. The periprocedural risk of stroke, incidence and degree of paravalvular leakage, procedure-related conduction block with the need for consecutive pacemaker implantation and a possible interference with coronary flow impose substantial risks to TAVI procedures.
As a next-generation device, the JenaValve™ system (JenaValve Technology GmbH, Munich, Germany) holds promise to reduce at least some of these TAVI-specific risks due to its unique stent design.
Technical concept of the JenaValve™ and clinical results
The JenaValve™ transapical system consists of a full porcine root valve mounted on a low-profile self-expanding nitinol stent (Figure 1). In contrast to devices expanding within the aortic annulus, the JenaValve™ relies on an active clip fixation of the native aortic valve leaflets, thereby eliminating great radial forces on cardiac and aortic structures. This allows for a short stent design that prevents coronary compromise by the native leaflets or stent struts, and that does not interfere with future coronary intervention. The feature of anatomically aligned positioning eliminates the need for rapid pacing during implantation. The porcine root leaflets are connected to flexible stent posts designed to reduce leaflet stress in diastole. The transapical device underwent successful animal testing7, a first-in-man implantation series8 and a pivotal study for CE-mark approval conducted in seven German centres9.
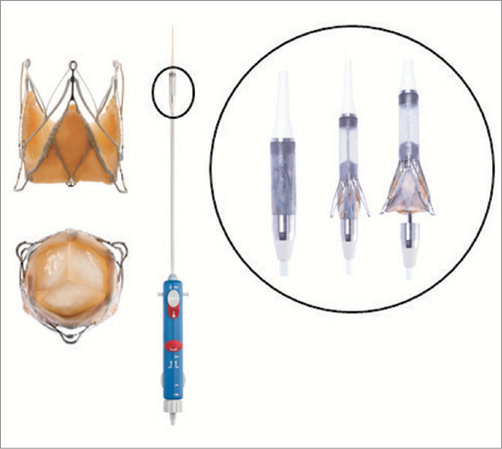
Figure 1. The JenaValve™ system. Porcine root valve mounted on self-expandable nitinol stent and delivery system.
JENAVALVE™ IMPLANTATION TECHNIQUE
The JenaValve™ prosthesis is manufactured in three different sizes (23 mm, 25 mm, and 27 mm) for implantation in native aortic annuli ranging from 21 mm to 27 mm in diameter. A sheathless 32 Fr delivery catheter is utilised for the three-step deployment procedure. After apical puncture and preprocedural balloon valvuloplasty using a balloon close to annular size, the delivery catheter with the loaded valve prosthesis is introduced over the wire into the left ventricle and advanced through the native valve annulus into the ascending aorta. Valve deployment is performed under fluoroscopic control under beating heart conditions without rapid pacing. At first, the positioning feelers are released and verified for anatomical orientation. By pulling back the catheter the positioning feelers are placed into the sinuses of the native aortic root. Correct positioning is controlled by two fluoroscopic views in different angulations (Figure 2, step 1). As a second step, the lower part of the stent is released, thereby clipping and attaching the native leaflets to the device and simultaneously unfolding the porcine valve (Figure 2, step 2 ). As a third step, the upper part of the stent is opened and the device is completely deployed (Figure 2, step 3). Device function is assessed by TEE and aortography before apical purse-string sutures are closed. In case of elevated transvalvular gradients or significant paravalvular leakage, re-balloon valvuloplasty can be performed with good results.
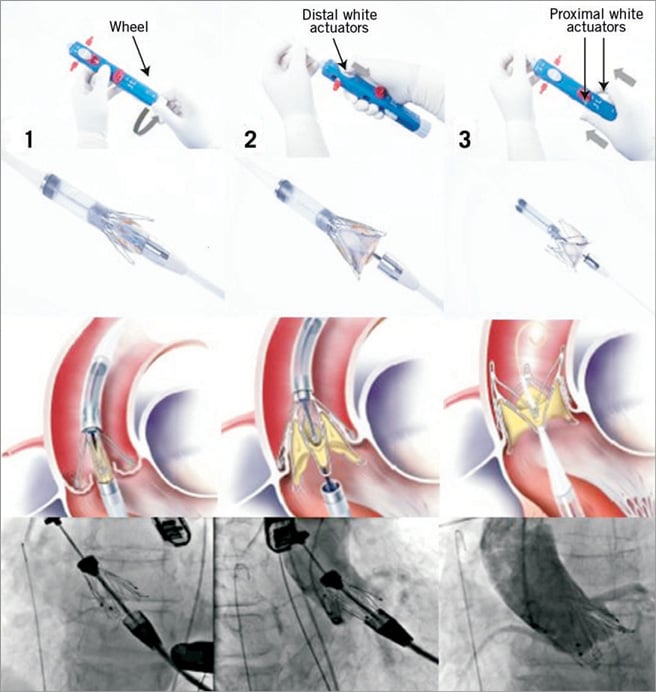
Figure 2. Procedural steps: Step 1. Release of positioning feelers by turning of the lower wheel and anatomical correct orientation and positioning in native sinuses under floursocopy; Step 2. Distal white actuator releases lower part of the stent, thereby actively fixating the native leaflets; Step 3. Pushing on the proximal white actuators releases the upper part of the stent and opens the stent completely before removal of the delivery system. All steps are carried out without rapid pacing.
STUDY RESULTS
Between October 2010 and July 2011, 73 patients (mean logistic EuroSCORE 28.4±6.5%) were enrolled in the pivotal CE-mark trial in seven German centres. Thirty-day results of this study have been published recently9. Transapical (TA) implantation of the JenaValve™ prosthesis was successful in 60 of 67 patients (procedural success rate 89.6%). In successfully treated patients, transvalvular pressure gradients significantly decreased from a mean of 40.6±15.9 mmHg pre-procedure to 10.0±7.2 mmHg post-procedure (p<0.0001). The effective orifice area (EOA) increased from a mean of 0.7±0.2 cm² to 1.7±0.6 cm² (p<0.0001). Post procedure, the majority of patients showed either no (AR 0, 47.5%) or minor (AR ≤1+, 39.0%) paravalvular leakage and aortic regurgitation (AR), while moderate AR 2+ was found in 13.6% of patients. No patient showed severe AR >2+. Coronary ostia were obstructed in none of the procedures. All-cause mortality at 30 days was 7.6% (5/66 patients) and two patients developed major cerebrovascular events (3.0%). New onset AV block and/or left bundle branch block requiring pacemaker implantation occurred in six patients (9.1%).
At six months, transvalvular gradients, left ventricular ejection fraction, effective orifice area (EOA) and paravalvular leak rates remained stable in comparison to early hospital outcome (Figure 3). Due to the high-risk patient profile with significant comorbidities six-month survival decreased to 72.7% while freedom from cardiac death was still 86.6% (Figure 4). At six months post procedure, the majority of patients showed sustainable clinical improvement as expressed by a favourable reduction in New York Heart Association (NYHA) Class (Figure 5).
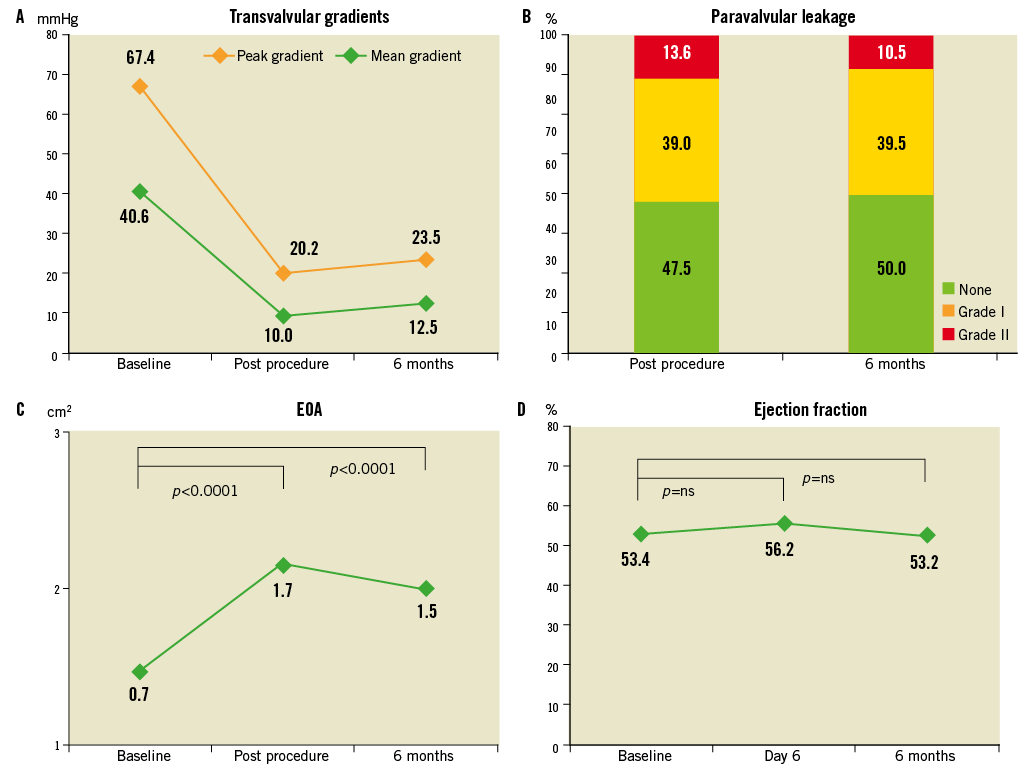
Figure 3. Echocardiographic outcome variables post procedure and at six months: transvalvular gradients [mmHg] (A), paravalvular leaks post procedure [%] (B), change in pre- and post-procedure EOA [cm²] (C), and ejection fraction [%] (D).
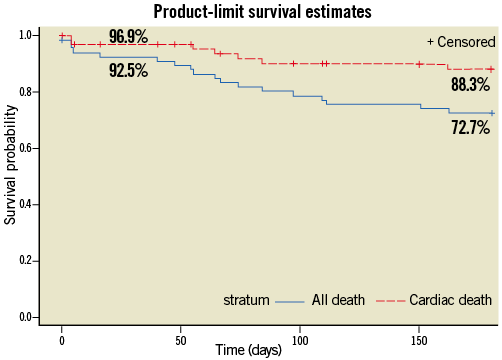
Figure 4. Kaplan-Meier survival at 30 days and 6 months.
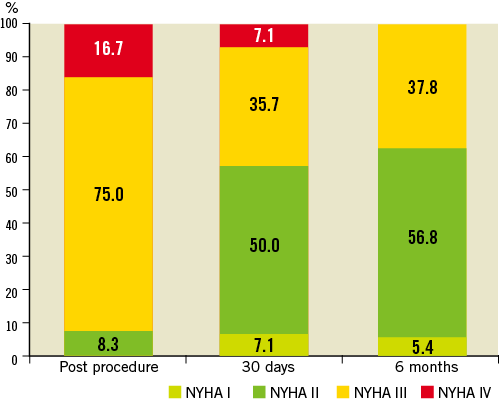
Figure 5. Change in NYHA Functional Class from baseline to 6 months follow-up.
Discussion
Despite all the improvements in procedural and clinical performance, transcatheter aortic valve implantation at present remains limited to the elderly inoperable or high-surgical-risk patient due to the elevated periprocedural risk of the procedure and unknown long-term outcome. Post-procedural paravalvular leakage with subsequent aortic regurgitation (AR) is one of the unsolved and frequent problems after TAVI using first-generation devices. In a recent publication of the PARTNER (Placement of AoRTic TraNscathetER Valve) trial cohort A two-year data, a randomised comparison of TAVI and surgical AVR, the authors showed a significant increase in mortality for patients with either mild, moderate or severe AR in comparison to patients with none or only trace AR10. Although AR is only mild in the majority of patients after TAVI, significant AR ≥2+ can be found in 17-25% of patients11-13. In contrast to the Edwards SAPIEN and Medtronic CoreValve™ prostheses, the JenaValve™ prosthesis achieves anatomically correct position by actively attaching to the native leaflets. This clip mechanism allows for a low stent profile and reduces paravalvular leakage. Therefore, the majority of patients in our series (86.5%) showed no or only mild paravalvular regurgitation (≤1+) while no patient developed significant post-procedural AR (>2+). The three-step implantation procedure is subject to a learning curve. The initial procedural success rate seen in the CE-mark trial prompted changes to the delivery catheter facilitating the ease of use. Post-market registry data and longer-term follow-up have to be awaited to show a positive impact of reduced paravalvular leakage on survival in patients treated with the JenaValve™ TAVI system.
Retrograde transfemoral (TF), transsubclavian (TS) or transaortic (TAo) TAVI is limited by the degree of calcification of the access vessels, the aorta and the aortic root. In contrast, transapical TAVI can be safely performed in almost every patient as long as the apex is not diseased. Higher complication rates, mainly due to the higher risk profile of TA versus TF patients, and risk for apical bleeding are often reported for transapical TAVI. Nevertheless, the transapical access route allows for a direct, antegrade delivery of the valve and avoids passage of the often calcified aortic arch and peripheral vessels. On-going experience with TA TAVI in the PARTNER trial cohort A revealed a significant influence of early learning curves on the procedural safety for the transapical access. While in the initial publication stroke rates were significantly higher in TA versus TF patients, publication of the non-randomised continuous access cohort revealed a distinct reduction in stroke rates for TA patients from 7.0% (initial PARTNER cohort A) to 2.0%14. In a meta-analysis of 53 studies including more than 10,000 patients treated with TAVI, Eggebrecht reported different stroke rates with different approaches and valve prostheses. Stroke rates appeared to be lowest in transapical Edwards SAPIEN patients vs. transfemoral Edwards SAPIEN patients (2.7±1.4% vs. 4.4±2.2%; 30-day stroke/TIA) although the difference did not reach statistical significance15. The JenaValve™ is designed for antegrade transapical delivery at the beating heart. Periprocedural stroke rate in the CE-mark study was 3% which seems to be low with regard to the very sick patients included with a mean logistic EuroSCORE of 28.4±6.5%. Nevertheless, thorough evaluation of further studies and registry data is needed before a positive influence of the transapical access route on stroke rates can be declared. Severe apical bleeding complications requiring circulatory support occurred in only 3% of patients in our series and have become a very rare complication in experienced centres.
Porcine root valves as used in the JenaValve™ prosthesis have been used for more than 30 years and have proven good long-term durability after surgical valve replacement16-18. The same valve used in this device is commercially available as a stentless (elan™ valve; Vascutek, Inchinnan, Renfrewshire, UK) and stented (aspire™ valve; Vascutek) biologic prosthesis for surgical AVR. Long-term follow-up on patients after implantation of the aspire™ valve revealed a freedom from structural valve degeneration of 98±2% after ten years19. Very good durability is also reported for bovine pericardium as used in the Edwards SAPIEN prosthesis; nevertheless, the heavy forces during valve crimping are known to leave structural damage in the connective tissue of the leaflets20. It is as yet unknown if this circumstance will negatively influence long-term functionality. Loading of the JenaValve™ prosthesis on the delivery catheter reduces leaflet tissue stress since the valve is only folded into a housing of 32 Fr diameter, potentially improving durability.
In the CE-mark study new onset of conduction disorders was only detected in 6/66 patients (9.1%), a rate that compares favourably to data reported for the Medtronic CoreValve™ which is also based on a self-expanding nitinol stent. Pacemaker implantation rates using the Medtronic CoreValve™ are reported to be as high as 30%21. Due to the low stent profile and leaflet clip mechanism, the nitinol stent of the JenaValve™ does not reach the left ventricular outflow tract and reduces radial forces in the annulus.
For the time being it is not possible to answer the question as to which TAVI device should be used in a particular patient. Nevertheless, the JenaValve™ system offers distinct features that may potentially address TAVI-specific problems better than competitors and may therefore be an attractive alternative treatment device for patients at high risk for surgical aortic valve replacement.
Conflict of interest statement
H. Treede and S. Ensminger are consultants for JenaValve Technologies. M. Ferrari and H. Figulla are shareholders and consultants for JenaValve Technologies. The other authors have no conflicts of interest to declare.

