According to the Euro Heart Survey II on valvular heart disease1, 7.4% of patients with single left-sided valve heart disease have native aortic regurgitation (AR). AR is often accompanied by an aneurysm or dilatation of the ascending aorta. In these conditions transcatheter aortic valve implantation (TAVI) remains a therapeutic challenge, since the absence of aortic valve calcification can result in inadequate anchoring of the stent frame, which could therefore result in subsequent valve embolisation or relevant paravalvular regurgitation2.
The JenaValve Trilogy (JenaValve) is a self-expanding valve with a supra-annular porcine valve sutured on a nitinol stent. In May 2021, it became the first device to receive the European Conformity (CE) mark, approving it for use in patients with AR. The key features of the JenaValve Trilogy System are the 3 radial locators that align the valve with the native aortic valve cusps. This locator-dependent anatomical alignment limits protrusion into the left ventricular outflow tract and the left ventricle.
An 83-year-old, highly symptomatic female patient with severe AR and high surgical risk was scheduled for TAVI by the local heart team. Her past medical history included coronary artery disease with previous stenting, frailty, arterial hypertension, and chronic kidney disease. The EuroSCORE II was 13.9%. Transthoracic and transoesophageal echocardiography revealed a mildly reduced left ventricular (LV) ejection fraction with severe central aortic regurgitation type I due to annular dilatation (vena contracta width of 9 mm and pressure half-time of 195 ms). A multidetector computed tomography (CT) scan, performed in preparation for the TAVI, showed a large aortic aneurysm with a maximum diameter of 62 mm, with an almost normal sized aortic annulus area of 453.4 mm² (Figure 1A-Figure 1E). The distance from the aortic annulus to the left coronary artery was 12 mm and 11 mm to the right coronary artery.
The elective procedure was performed under conscious sedation. The 25 mm JenaValve device was implanted at the annulus via a transfemoral approach (Moving image 1-Moving image 5). After valve deployment, the locators clipped onto the native leaflets, securing the valve safely to address dislocation concerns. Furthermore, increased sealing was provided by the clipping mechanism, in addition to a distal sealing ring.
Post-implantation fluoroscopy revealed an appropriate device position. Moreover, quantitative aortography (CAAS-A Valve 2.1.2; Pie Medical Imaging) for quantification of the regurgitation fraction from the aortogram showed an AR fraction of 3% (Figure 1F). The patient was discharged without complications and with significant functional improvement (New York Heart Association Functional Class I). To our knowledge, this is the first report of a JenaValve implantation in an ascending aortic aneurysm, indicating the potential of this valve device in complex anatomical settings. With regard to the ascending aortic aneurysm, the major concerns were the increased vulnerability of the aortic wall to the device passage and the delivery system housing. Additionally, the JenaValve Trilogy System requires the passage of a long 18 Fr sheath into the sinotubular junction, so it was anticipated that manipulation had to be undertaken very carefully. Procedurally, the sheath was then withdrawn into the descending aorta after the introduction of the delivery catheter into the aortic sinus, which required careful manipulation. Once the system was in place, the necessary catheter flexing and rotation to position the locators into the nadir of the aortic cusps could be performed without further interaction with the ascending aortic aneurysm by using wire manipulations to centralise the device. Fortunately, by flexing the system, extreme wire pushing and subsequent interaction with the outer aortic curvature were prevented.
The unique implant mechanism of the JenaValve device offered an appealing option for this patient. Currently, the JenaValve device is being evaluated globally in a prospective, multicentre, non-randomised, single-arm, open-label clinical study to demonstrate its safety and effectiveness (The JenaValve ALIGN-AR Pivotal Trial; ClinicalTrials.gov: NCT04415047).
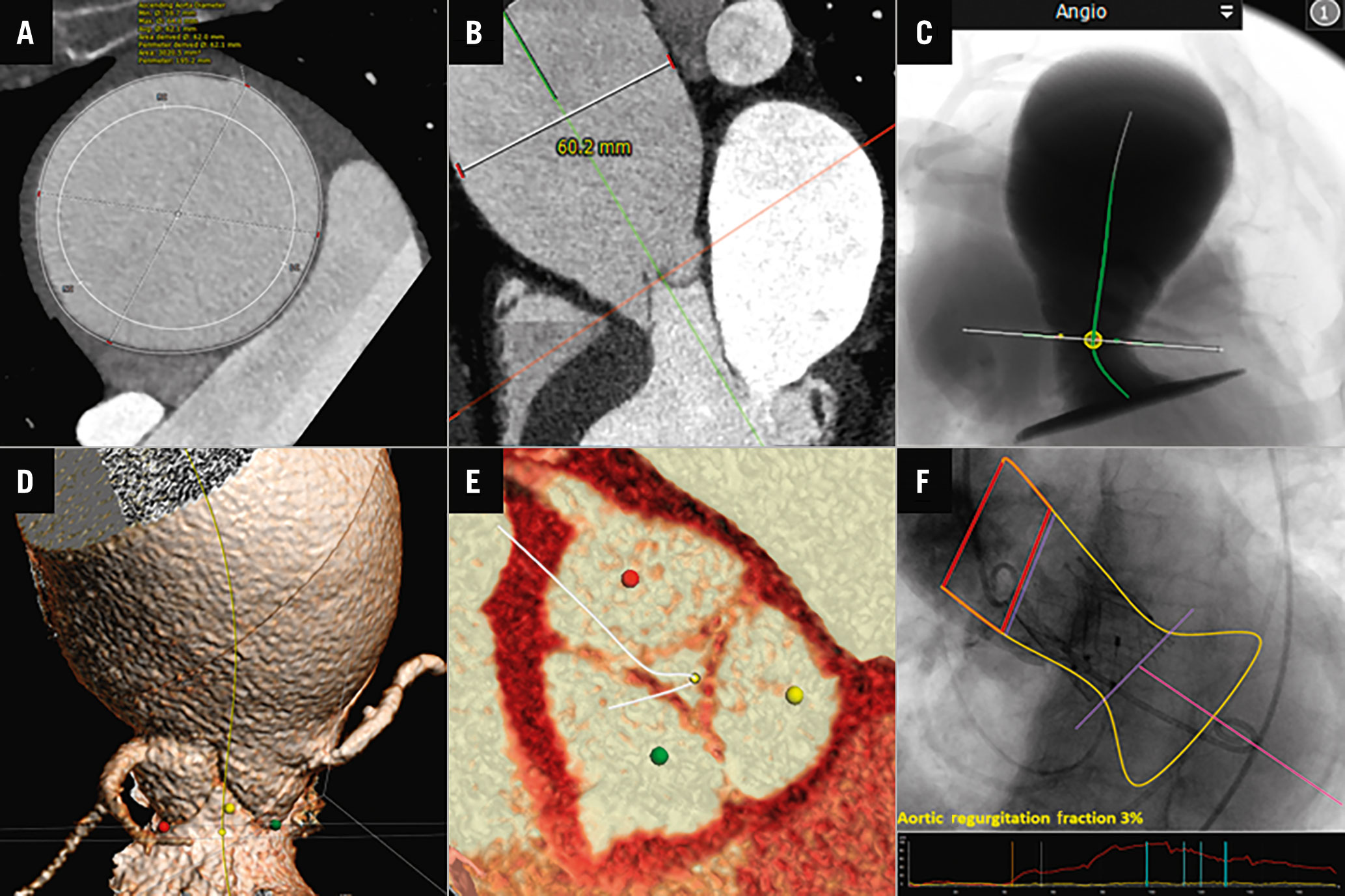
Figure 1. CT and angiographic case visualisation. A) CT angiography (CTA) showing an axial image of an ascending aortic aneurysm with a maximum diameter of 62 mm. B) CTA multiplanar reconstruction view of aortic root and ascending aorta. C) CTA simulation showing left ventricular outflow tract (LVOT), aortic annulus and ascending aortic aneurysm. D) 3D volume rendering technique long-axis view of LVOT, aortic annulus, coronaries, and ascending aorta. E) 3D-CTA short-axis view of aortic valve with zero calcium score. F) Quantitative aortography analysis of aortic regurgitation after transcatheter aortic valve implantation using videodensitometry.
Conflict of interest statement
E. Kuhn reports consultancy/personal fees from Medtronic, Edwards Lifesciences, Boston Scientific, and Abbott. J.M. Sinning is proctor for Medtronic and Boston Scientific; and received research grants, consultancy fees, and speaker honoraria from Boston Scientific, Edwards Lifesciences, and Medtronic. S. Baldus reports lecture fees from JenaValve and lecture and speaker fees from Edwards Lifesciences. P.W. Serruys reports consultancy/personal fees from Philips/Volcano, SMT, Novartis, Xeltis, and Meril Life. M. Adam reports grants and personal fees from Medtronic, and personal fees from JenaValve, Edwards Lifesciences, and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Aortography before transcatheter aortic valve implantation shows an ascending aorta aneurysm.
Moving image 2. Aortography confirms severe native aortic regurgitation (AR) and pigtail position in left ventricle.
Moving image 3. Control aortography during JenaValve positioning.
Moving image 4. Release of the JenaValve.
Moving image 5. Post-aortography shows stable valve position with trace AR.

