
Introduction
Transcatheter aortic valve implantation (TAVI) is now well established in the contemporary management of severe aortic stenosis (AS) with a multitude of valve designs available. The transapical (TA) J-Valve™ system (JC Medical Inc., Burlingame, CA, USA) has previously been shown to be effective for the treatment of both severe AS and aortic regurgitation (AR)1,2.
The J-Valve transcatheter aortic valve consists of the valve and three U-shaped “anchor rings” (Figure 1A) and is deployed in a two-step process. The transfemoral (TF) valve is delivered by an 18 Fr steerable delivery system (Figure 1B). First, the anchor rings are opened above the native valve and are retracted (TA) or advanced (TF) into the valve apparatus allowing automatic anatomic alignment in the aortic sinuses and clasping of the native valve leaflets (Figure 1C). This can be visualised on fluoroscopy. Once positioned, the self-expanding valve is then deployed within the anchor rings and secures the native valve leaflets (Figure 1D). The valve, which is not recapturable, is currently available in three sizes (Figure 1E). We present the first-in-human experience of the TF J-Valve system for the treatment of severe AR.
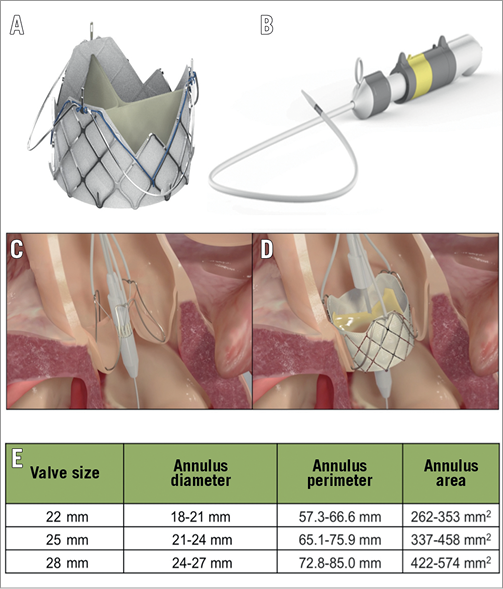
Figure 1. Transfemoral J-Valve system. A) Valve consisting of bovine pericardial leaflets within a nitinol stent frame deployed within a nitinol anchor ring. B) J-Valve steerable transfemoral delivery system. Outer diameter of 18 Fr with minimal femoral access diameter of 5.5 mm. C) Illustration of anchor ring deployment within native valve cusps with anatomic alignment and leaflet grasping. D) Deployment of self-expanding J-Valve within anchor ring. E) Valve sizing chart. A 31 mm valve is also in development.
Methods
A 42-year-old male with prior subaortic membrane repair presented with recurrent heart failure hospitalisations due to severe AR (New York Heart Association [NYHA] Class IV) and high diuretic requirements. Echocardiography revealed normal left ventricular (LV) systolic function, an LV end-diastolic diameter of 66 mm and a non-calcified tricuspid aortic valve with severe AR (Figure 2A). Invasive cardiac catheterisation revealed normal coronary arteries and raised LV end-diastolic pressure of 35 mmHg. The patient had multiple comorbidities including morbid obesity (BMI >50 kg/m2), diabetes and stage IV chronic kidney disease (eGFR 29 ml/min per 1.73 m2). The Society of Thoracic Surgeons (STS) predicted risk of mortality was 7.8% and the patient was deemed at prohibitive risk for surgical aortic valve replacement or TA TAVI by the multidisciplinary Heart Team. Preoperative valve sizing with computed tomography was not optimal due to elevated body mass index and attempts to reduce contrast load to lower the risk of acute kidney injury; therefore, intraoperative transoesophageal echocardiography (TEE) was performed to confirm sizing. TEE is not required for deployment of the valve.
Right common femoral arterial access was gained, and an 18 Fr sheath inserted. The aortic valve was crossed and a small Safari™ wire (Boston Scientific, Marlborough, MA, USA) was placed in the left ventricle. A 28 mm J-Valve system was advanced over the wire into the aortic root and deployed without the need for rapid pacing. Deployment of the valve proved challenging due to the small size of the sinotubular junction which interacted with the valve’s anchor rings; however, a stable position was attained. Following valve deployment, there was an immediate improvement in haemodynamics. Echocardiography and aortography revealed no significant AR; however, the aortic portion of the valve frame appeared underexpanded on fluoroscopy (Figure 2B-Figure 2D). We believe that this was due to rotation of the delivery system following deployment of the anchor rings, but prior to valve deployment, resulting in the twisting of suture connections at the top of the valve. Mean transaortic gradient on transthoracic echocardiography (TTE) was 39 mmHg with no AR. Post-dilatation of the TAVI valve was subsequently performed with a 25 mm balloon, resulting in full expansion of the valve frame and a reduction of the mean gradient to 16 mmHg (Figure 2E, Figure 2F).
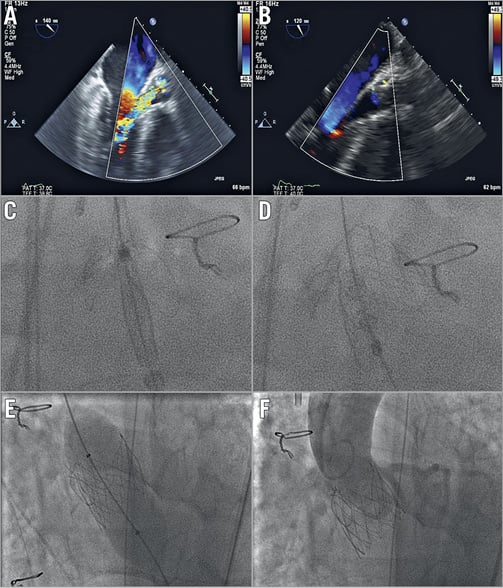
Figure 2. Transoesophageal echocardiography (TEE) and fluoroscopy of J-Valve deployment. A) TEE pre deployment showing severe aortic regurgitation. B) TEE post deployment showing trivial paravalvular aortic regurgitation. C) Fluoroscopy of anchor deployment into native valve cusps. D) Fluoroscopy of deployed valve into anchor rings. E) Post-dilatation of valve with 25 mm non-compliant balloon. F) Final aortogram showing well deployed valve with no aortic regurgitation.
Results
The patient remained intubated for eight days due to high respiratory support requirements and required temporary intermittent haemodialysis. On discharge, the patient was euvolaemic, had NYHA Class II symptoms, improved renal function (eGFR >50) and no diuretic requirements. At 30-day follow-up the patient remained in NYHA Class II and off diuretics. TTE revealed a mean gradient of 18 mmHg with no AR and normal LV systolic function.
Discussion
The J-Valve TF system offers potential for the treatment of severe AS and pure AR. Despite a difficult post-procedural course, in this case the system delivered a promising clinical result. Underexpansion of the valve, as occurred in this case, can be prevented by avoiding rotation of the delivery system after deployment of the anchor rings. Changes to the delivery system are also being made to address this issue. A TF approach is less invasive than TA access whilst preserving the leaflet-securing and anatomic positioning abilities of the J-Valve system. These leaflet-securing properties offer possible advantages in the treatment of pure AR and may also offer a solution to the treatment of patients with either native AS or bioprosthetic valve failure at high risk of coronary obstruction, since the anchor rings may retract the native or bioprosthetic valve leaflets to avoid obstructing the coronary ostia3. This requires further investigation. Additionally, to date the valve has demonstrated a low pacemaker rate of approximately 5%1,2, probably due to the relatively high deployment within the anchor rings.
Limitations
This is a single case study. The TF J-Valve system requires validation with further clinical data and large-scale clinical trials.
Conclusions
The TF J-Valve system is a novel transcatheter heart valve system with leaflet-securing anchor rings. It may be well suited to patients with non-calcified aortic valve disease and those at risk of coronary obstruction. Further clinical experience is required.
| Impact on daily practice The TF J-Valve is not currently available outside of clinical studies; however, it is a potentially advantageous device in certain clinical situations. |
Conflict of interest statement
P. Blanke, J. Leipsic, D. Wood, and J. Webb are consultants to and receive research support from Edwards Lifesciences. A. Cheung is a consultant to Abbott Vascular, Medtronic and Neovasc. J. Ye is a consultant to Edwards Lifesciences and JC Medical. The other authors have no conflicts of interest to declare.

