CASE SUMMARY
BACKGROUND: An 87-year-old man was treated with a JenaValve™ due to severe symptomatic aortic stenosis. During implantation, the feelers of the JenaValve could not be positioned correctly inside the corresponding aortic sinus.
INVESTIGATION: Transapical aortic valve implantation, angiography of the aortic root, 3D transoesophageal echocardiography.
DIAGNOSIS: Incorrectly positioned JenaValve.
MANAGEMENT: “Snare and guide” the feelers into the correct aortic sinus.
KEYWORDS: bioprosthesis, JenaValve™, minimally invasive, transcatheter aortic valve implantation (TAVI), transapical aortic valve implantation
Abbreviations
CABG: coronary artery bypass graft
CAU: caudal
CCS: Canadian Cardiovascular Society
LAO: left anterior oblique
LIMA: left internal mammary artery
NYHA: New York Heart Association
PAOD: peripheral arterial occlusive disease
RAO: right anterior oblique
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
PRESENTATION OF THE CASE
An 87-year-old male patient was referred to hospital due to severe symptomatic aortic stenosis. He suffered from dyspnoea NYHA III and angina pectoris CCS II; the occurrence of syncope was denied. Transthoracic echocardiography showed a preserved left ventricular ejection fraction (LVEF) of 60%, moderate left ventricular hypertrophy with diastolic dysfunction grade I and mild pulmonary hypertension. The aortic valve opening area was measured as 0.7 cm2 with a mean pressure gradient of 45 mmHg. The patient had undergone successful CABG surgery 14 years previously (LIMA ad LAD, SVB ad M2). Moreover, he suffered from peripheral arterial occlusive disease (PAOD) with amputation of the right lower leg and was further treated with an endovascular aortic stent graft due to abdominal aortic aneurysm. The logistic EuroSCORE was 34.15%, and the STS score was 23.41%.
The preoperative coronary angiography revealed a severe stenosis of the SVB to the marginal branch, which was treated successfully by implantation of a drug-eluting stent. Angiography of the aortic root revealed a tricuspid aortic valve with slightly dilated non-coronary sinus; the size of the other sinus and the ascending aorta was within the normal range. The distance to both coronary ostia was more than 10 mm.
The case was discussed in our interdisciplinary “Heart Team”. Based on the age and severe concomitant diseases, the Heart Team decided to perform a transcatheter aortic valve implantation via the transapical approach as severe PAOD and abdominal aortic stent implantation made the transfemoral approach unfeasible. Due to the anatomy of the aortic bulbus and an annulus diameter of 23.5 mm measured by 3D transoesophageal echocardiography, a JenaValve™ 25 mm (JenaValve Technology GmbH, Munich, Germany) was chosen1,2.
The procedure was performed in a fully equipped hybrid operating room under general anaesthesia. After administration of unfractionated heparin (100 IU/kg) a 6 Fr sheath was inserted via A. radialis dexter and a 6 Fr pigtail catheter, 110 cm (Cordis, Johnson & Johnson, Warren, NJ, USA), was guided into the non-coronary sinus. Angiography of the aortic root was performed and the three sinuses were orientated in a perpendicular angle (Figure 1, Moving image 1).
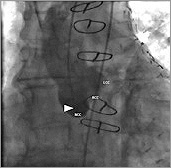
Figure 1. Angiography of the aortic root (RAO 13°, CAU 11°). Non-coronary cusp (NCC), right coronary cusp (RCC) and left coronary cusp (LCC) are in a perpendicular plane to the aorta. The tip of the pigtail catheter (![]() ) is positioned in the NCC, which is slightly dilated.
) is positioned in the NCC, which is slightly dilated.
After that, the apex of the heart was prepared through a left anterolateral mini-thoracotomy. An epicardial pacing wire was placed and tested for effective “rapid ventricular pacing”. Three apical purse-string sutures were placed close to the apex and lateral to the left anterior descending coronary artery. Thereafter, the apex was punctured with a needle and a guidewire (EMERALD™ 150 cm, 0.035”; Cordis, Johnson & Johnson) was inserted anterogradely across the aortic valve and placed in the descending aorta. Via a 5 Fr Judkins right catheter, 100 cm (Cordis, Johnson & Johnson), the guidewire was exchanged for a stiff guidewire (Amplatz Extra Stiff Wire, 260 cm; Cook Medical, Bloomington, IN, USA). After that, a balloon valvuloplasty was performed (sheathless) under rapid ventricular pacing using a 22×40 mm Ballon (VACS II; Dr. Osypka GmbH, Rheinfelden-Herten, Germany), filled with 1:10 diluted contrast medium.
The balloon catheter was retrieved and the 32 Fr Cathlete delivery system (JenaValve Technology GmbH) was inserted over the extra stiff wire. The JenaValve was placed into the ascending aorta under fluoroscopic guidance. Subsequently, the three “positioning feelers” were released and the valve was pulled back and rotated so that each feeler was in the corresponding aortic sinus. The correct position was controlled by angiography and visualisation by a free “swing” of each feeler in synchrony to each heartbeat.
Even after several attempts at changing the angle of the delivery system, the feeler of the left coronary sinus could not be positioned correctly and was always placed beneath the aortic annulus (Figure 2, Figure 3, Moving image 2, Moving image 3). What would you do next?
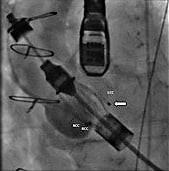
Figure 2. Angiography of the aortic root (LAO 30°, CRA 19°). JenaValve 25 mm inside the native aortic plane, white arrow (![]() ) indicating the “left coronary” feeler positioned outside the LCC.
) indicating the “left coronary” feeler positioned outside the LCC.
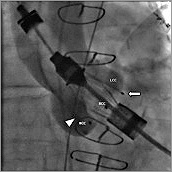
Figure 3. Angiography of the aortic root (RAO 13°, CAU 11°). The “left coronary” feeler (![]() ) is still positioned outside the LCC. The tip of the pigtail catheter (
) is still positioned outside the LCC. The tip of the pigtail catheter (![]() ) is located in the NCC.
) is located in the NCC.
How would I treat?
THE INVITED EXPERT’S OPINION
The authors describe a symptomatic AS patient at an advanced age, with prior CABG and extensive peripheral vascular disease, total logistic EuroSCORE 34%.
Due to the prohibitive surgical risk, this patient has a clear Class I recommendation for TAVI (level of evidence B), according to the recently published 2014 AHA/ACC Guideline3. A transfemoral approach was not feasible. Prior CABG with a patent LIMA graft made a transaxillary or direct aortic approach unfavourable. For transapical TAVI, multiple antegrade devices are currently available with variable designs and (more importantly) variable positioning, deployment, and fixation features. In contrast to intra-annular fixation, the JenaValve™ prosthesis with porcine root valve and nitinol stent anchors itself with a clip mechanism on the native aortic valve leaflets4. Accurate, anatomical positioning is an absolute must. The JenaValve, therefore, contains three positioning feelers, which should be positioned deep into the sinus of the native aortic root. At first, orientation is checked by carefully rotating the system, making sure one of the feelers is oriented strictly anteriorly for the right coronary cusp. The valve retainer should be placed distally enough to allow the feelers, once released, to be pulled deep into the sinus at the aortic side of the stenosed and/or insufficient native valve. A narrow sinotubular junction (STJ) may complicate this pullback manoeuvre, and care must be taken not to injure the aortic wall. Therefore, feeler release is advised at the level of the distal aortic root, but must of course be distal to the free edge of each native leaflet to ensure adequate capture. Coaxial positioning prior to pullback facilitates positioning of all three feelers in each individual sinus. Hence, a bicuspid valve is contraindicated for the JenaValve.
The authors repeatedly experienced inadvertent positioning of the “left coronary” feeler outside the left coronary cusp (LCC), i.e., infra-annular. At first, one must be sure that the feeler is truly infra-annular and angiography is not skewed by a non-perpendicular view. An even flaring out of all three feelers, while “swinging” on each heartbeat, suggests accurate positioning. A feeler that is inadvertently positioned inside the annulus will not open up, and must be checked for upon rotational fluoroscopy prior to final valve release. Considerable “induced” aortic regurgitation also suggests inaccurate positioning, since an infra-annular feeler will prevent leaflet coaptation prior to full release. In case of malpositioning, the device should be advanced, re-oriented, and pulled back again while obtaining a coaxial annular alignment by manipulating the stiff wire. At this stage, the JenaValve is still fully retrievable. So, the feelers may be closed (of course well above the free edge of the native leaflets to prevent accidental capture), safely re-oriented, and released again for a new pullback manoeuvre. In case of consequent malalignment in the LCC, tension on the stiff wire may help to align the device on the inner curvature of the ascending aorta. Although not to be advised, inadvertent release of one of the three clips infra-annular did not result in paravalvular leakage, or valvular dysfunction, or early or late migration in our early JenaValve experience.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION
Ibrahim et al describe an interesting complication during a transapical aortic valve implantation (TA-AVI) using the JenaValve™, a second-generation transcatheter heart valve (THV). The JenaValve has a unique fixing mechanism including three clips. These are clipped onto the native aortic valve cusps after anatomical placement of its three feelers into the aortic valve sinuses2. This fixing mechanism has not only resulted in very low rates of post-implantation paravalvular leakage5, but also led to CE-mark approval of the JenaValve for treatment of patients with pure aortic regurgitation4. Nevertheless, as a consequence of this fixing mechanism, this THV should only be used in patients with tricuspid aortic valves.
The authors were unable to place one of the three feelers of the prosthesis adequately into the left coronary sinus. From the case report, it is unclear why that was the case. This complication has not been described in the CE-mark trial or the Jupiter Registry5. From the images provided, the valve seems to sit adequately in the aortic root and the authors mention that they assessed the anatomy of the native aortic valve using a root angiogram before the procedure. I personally would have preferred additional 3D imaging assessment prior to the procedure using echocardiography or cardiac computed tomography, as these techniques provide better reassurance that the native aortic valve truly has three normal sinuses.
Nevertheless, in the situation described, placement of the third feeler into the left coronary sinus was impossible despite the multiple attempts of the highly experienced team involved. Two bail-out strategies come to mind. The first would be an additional attempt to facilitate placement of the feeler. I would have placed an additional pigtail catheter into the left coronary sinus, which would possibly further close the left coronary cusp and could have facilitated easier placement of the third feeler.
In case placement of the JenaValve was still impossible, one could have used an additional feature of the JenaValve, namely the ability to retrieve the prosthesis even at this point in the procedure. I would have brought the feelers back into the catheter housing of the JenaValve and exchanged it for a standard 22 Fr Ascendra sheath (Edwards Lifesciences, Irvine, CA, USA), feasible for deploying a 26 mm SAPIEN XT THV (Edwards Lifesciences). The reason why I would use the larger sheath of the SAPIEN XT and not the smaller introducer device for a SAPIEN 3 THV is that the sheathless JenaValve system has a diameter of 32 Fr and therefore the hole in the apex is more likely to be adequately occluded during the second TA-AVI using the rather larger sheath size.
I look forward to seeing the approach the authors used in this situation to facilitate a successful TA-AVI procedure and would like to thank them for sharing their experience.
Conflict of interest statement
The author is a consultant for JenaValve, Principal Investigator of the JenaValve Jupiter Registry, consultant for Edwards Lifesciences, and Principal Investigator of the Edwards Lifesciences SOURCE Registries.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The 6 Fr pigtail catheter was guided inside the left coronary sinus. As this was difficult, the pigtail catheter was stiffened with a guidewire to facilitate the positioning. Angiography confirmed the incorrect location of the feeler (Figure 4, Moving image 4). After that the valve was pulled back several times to achieve a correct position of all feelers, but without success. Therefore, the left coronary feeler was caught with the pigtail catheter in order to direct it into the left coronary sinus. This manoeuvre was effective, so that finally all three feelers were inside the corresponding aortic sinus and location was checked by another angiography (Figure 5, Figure 6, Moving image 5, Moving image 6). Surprisingly, the pigtail catheter that was around the “left coronary” feeler was stuck and could not be retrieved. Only the unfolding of the tip of the pigtail catheter by an inserted guidewire (EMERALD™ 150 cm, 0.035”; Cordis, Johnson & Johnson) and the simultaneous loosening of the valve delivery system led to the successful withdrawal of the pigtail catheter.
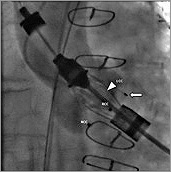
Figure 4. Angiography of the aortic root (RAO 13°, CAU 11°). The tip of the pigtail catheter (![]() ) is now located in the LCC.
) is now located in the LCC.
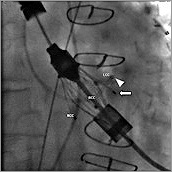
Figure 5. The “left coronary” feeler (![]() ) was caught with the tip of the pigtail catheter (
) was caught with the tip of the pigtail catheter (![]() ) and was guided into the LCC.
) and was guided into the LCC.
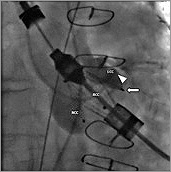
Figure 6. Angiography of the aortic root (RAO 13°, CAU 11°). The “left coronary” feeler (![]() ) is now correctly located in the LCC. The tip of the pigtail catheter (
) is now correctly located in the LCC. The tip of the pigtail catheter (![]() ) got stuck during this manoeuvre.
) got stuck during this manoeuvre.
After verifying the correct position of each feeler by angiography (Figure 7, Moving image 7), the valve was released and the delivery system was retrieved with the super stiff guidewire left inside the aorta. TEE and angiography showed moderate para-aortic insufficiency (Figure 8) so that a post-dilatation with a 23×40 mm balloon was performed, resulting in a mild para-aortic insufficiency (not shown). Correct valve position and function was confirmed by TEE with transvalvular gradients of maximum 9 mmHg and mean of 5 mmHg.
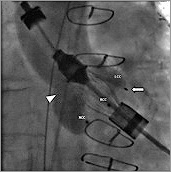
Figure 7. Angiography of the aortic root (RAO 13°, CAU 11°). All three feelers are now positioned in their corresponding cusps.
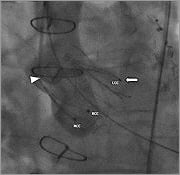
Figure 8. Angiography of the aortic root (RAO 13°, CAU 11°) shows correct position of the JenaValve 25 mm.
After wound closure the patient was transferred to the intensive care unit, was extubated without complications and was discharged seven days after the procedure in a good clinical condition.
Discussion
This case highlights the importance of preoperative diagnostic and technical considerations. First, due to the dilated coronary sinus, the selection of the valve might not be perfect. Maybe another type of valve (e.g., the Edwards SAPIEN XT 26 mm; Edwards Lifesciences) would have generated fewer difficulties. Secondly, a different puncture site of the transapical approach might have changed the angle towards the aortic root, so that positioning of the feelers would have been easier. At the time the JenaValve is released partially, it cannot be retrieved completely, so that every attempt to position the valve correctly has to be made in order to avoid open heart surgery.
Different possibilities to solve this problem were taken into consideration:-
– First, folding the super stiff wire in such a manner that the JenaValve is shifted towards the left coronary sinus with the possibility of positioning the feeler inside the left coronary sinus.
– Second, placing the pigtail catheter inside the left coronary sinus in order to guide the feeler along the pigtail catheter into the right position.
– Third, conversion to open heart surgery as long as the patient is haemodynamically stable. As the perioperative risk was high, this was only considered to be a bail-out option.
Finally, the pigtail catheter was used as a “guideway” and “snare” to the left coronary sinus. This can be difficult sometimes, especially when the A. radialis dexter is used as access. By stiffening the catheter with a guidewire inside (which can be folded at its tip), positioning may be facilitated. In the present case, we managed to guide the feeler into the correct aortic sinus. However, the pigtail catheter was stuck inside the valve, so that it could only be retrieved by stiffening the pigtail with simultaneous loosening of the traction of the valve. In the end, the valve was implanted successfully due to the interdisciplinary cooperation of the cardiac surgeon loosening the traction of the valve and the cardiologist stiffening and finally withdrawing the pigtail. This underlines the importance of an experienced Heart Team performing TAVI procedures together6.
Conflict of interest statement
U. Kappert works as a proctor for JenaValve. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Angiography of the aortic root (RAO 13°, CAU 11°). NCC, RCC and LCC are in a perpendicular plane to the aorta. The tip of the pigtail catheter is positioned in the NCC, which is slightly dilated.
Moving image 2. Angiography of the aortic root (LAO 30°, CRA 19°). JenaValve 25 mm inside the native aortic plane. The “left coronary” feeler is positioned outside the LCC. In contrast to the “right and non-coronary” feelers, the “left coronary” feeler shows no pulsatile movements.
Moving image 3. Angiography of the aortic root (RAO 13°, CAU 11°). The “left coronary” feeler is still positioned outside the LCC. The tip of the pigtail catheter is located in the NCC.
Moving image 4. Angiography of the aortic root (RAO 13°, CAU 11°). The tip of the pigtail catheter is now located in the LCC. The “left coronary” feeler is still positioned outside the LCC.
Moving image 5. The “left coronary” feeler was caught with the tip of the pigtail catheter and was guided into the LCC.
Moving image 6. Angiography of the aortic root (RAO 13°, CAU 11°). The “left coronary” feeler is now correctly located in the LCC. The tip of the pigtail catheter got stuck during this manoeuvre.
Moving image 7. Angiography of the aortic root (RAO 13°, CAU 11°) shows correct position of the JenaValve 25 mm.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Angiography of the aortic root (RAO 13°, CAU 11°). NCC, RCC and LCC are in a perpendicular plane to the aorta. The tip of the pigtail catheter is positioned in the NCC, which is slightly dilated.
Moving image 2. Angiography of the aortic root (LAO 30°, CRA 19°). JenaValve 25 mm inside the native aortic plane. The “left coronary” feeler is positioned outside the LCC. In contrast to the “right and non-coronary” feelers, the “left coronary” feeler shows no pulsatile movements.
Moving image 3. Angiography of the aortic root (RAO 13°, CAU 11°). The “left coronary” feeler is still positioned outside the LCC. The tip of the pigtail catheter is located in the NCC.
Moving image 4. Angiography of the aortic root (RAO 13°, CAU 11°). The tip of the pigtail catheter is now located in the LCC. The “left coronary” feeler is still positioned outside the LCC.
Moving image 5. The “left coronary” feeler was caught with the tip of the pigtail catheter and was guided into the LCC.
Moving image 6. Angiography of the aortic root (RAO 13°, CAU 11°). The “left coronary” feeler is now correctly located in the LCC. The tip of the pigtail catheter got stuck during this manoeuvre.
Moving image 7. Angiography of the aortic root (RAO 13°, CAU 11°) shows correct position of the JenaValve 25 mm.

