
Transcathether aortic valve implantation (TAVI) has been approved in patients with symptomatic severe aortic stenosis across all surgical risk profiles. As TAVI expands to younger and lower-risk patients, the prevalence of haemodynamically significant coronary artery disease (CAD) in patients with aortic stenosis undergoing TAVI is decreasing compared to their geriatric counterparts. However, younger patients with longer life expectancies have a greater cumulative risk of progression of CAD and acute coronary syndrome, requiring diagnostic angiography and therapeutic intervention. While considerable advances have been made in optimising procedural techniques to ameliorate limitations associated with the current TAVR technology, coronary access after TAVI remains an unaddressed problem. Furthermore, younger patients undergoing TAVI may in due course require valve reintervention for structural valve degeneration, with TAVI-in-TAVI being the less invasive option when anatomically feasible. The technical challenges associated with coronary re-access in the presence of a transcatheter heart valve (THV) are therefore becoming increasingly important, with the problem being further compounded in patients undergoing redo TAVI.
Considering the risk of coronary obstruction associated with redo TAVI in certain types of aortic root anatomy, these patients represent a somewhat self-selected population, with those having unfeasible root anatomy less likely to undergo redo TAVI1. There are three important technical challenges to consider during coronary re-access after redo TAVI, stemming from the inherent design of the index THV and its anatomic relationship with the coronary ostia after redo TAVI, making coronary access more challenging after redo TAVI compared to index TAVI. 1) Cylinder effect. In addition to the in situ barrier of native aortic valve leaflets, coronary re-access may be further impaired by a cylindrical cage created by vertical displacement of the leaflets of the first THV after redo TAVI. The height of the first THV leaflets may therefore impact significantly on the feasibility of coronary re-access after redo TAVI, particularly in supra-annular THVs with tall leaflets. 2) Cannulation through the THV stent frame. When the sinotubular junction (STJ) or coronary ostia are below the THV stent frame, one would need to traverse the stent frame to access the coronary artery. However, in cases where the STJ or coronary ostia are above the THV stent frame, coronary artery access can be performed relatively easily above the frame, rather than through the frame. Coronary re-access is therefore likely to be easier after intra-annular THVs with shorter stent frames. 3) Commissural misalignment. Unlike surgical aortic valve replacement (SAVR), in which consistent commissural alignment is possible, commissural alignment is not consistently feasible with current commercially available THVs2. This problem is further compounded by redo TAVI, with a greater possibility of both first and second THV commissural posts ending up in proximity to the coronary ostia and making coronary access more difficult.
In this issue of the Journal, Buzzatti et al provide insight into the potential risk of coronary access and obstruction after redo TAVI3.
Using an observational, retrospective cohort analysis, the investigators studied 221 patients who underwent computed tomography following TAVI with five different THVs. In addition to aortic root analysis, several anatomic considerations that may impact on the feasibility of coronary re-access after redo TAVI were evaluated, including final THV position relative to the annulus and coronary ostia, THV commissural alignment, valve-to-aorta distance (VTA) as the shortest distance between the THV frame and the aortic wall above the coronary ostium, and valve-to-coronary distance (VTC) as the distance between the THV frame and coronary ostium. In patients with coronary ostium below the THV commissure, the investigators defined VTA <2 mm as being an increased risk of impaired coronary access, and VTC <2 mm as being an increased risk of coronary obstruction. On the basis of this definition, 55.6% of patients had an increased risk of impaired coronary access, and 14.9% had an increased risk of coronary obstruction. Smaller and shorter STJs were identified as anatomic predictors of impaired coronary access after redo TAVI, while supra-annular THVs, THV oversizing, height of index THV leaflets and higher implantation depth were identified as procedural predictors. Severe commissural misalignment or THV commissure-coronary angle <20° was seen in 27.5% for the left main and 28.3% for the right coronary artery.
The predicted risk of impaired coronary access and coronary obstruction after redo TAVI in the current study was higher than that reported in prior studies4,5. This observed difference could be due to: a) differences in patient populations, with this study conducted in all-comers in elderly TAVI patients; b) smaller mean aortic root dimensions, again possibly due to older patients with smaller aortic root anatomies; c) differences in THV types, with over 70% of this study cohort comprising supra-annular THVs. Due to their inherent design, supra-annular THVs present challenges in coronary re-access, which is corroborated in the present study, along with the height of the first THV leaflets being the most important procedural predictor of impaired access. Coronary re-access is further compounded by the tall THV frame and the need to achieve a shallower implant depth to mitigate new conduction disturbances requiring a permanent pacemaker. Appropriate THV selection at index TAVI, as demonstrated in a recent study, remains crucial to anticipate the feasibility of coronary re-access and redo TAVI5.
In light of recent studies suggesting several anatomic and device-related factors impacting on coronary re-access after redo TAVI3,4,5,6, we propose a novel classification system to simplify the assessment of coronary re-access after redo TAVI (Figure 1). Four types of aortic root anatomy were conceptualised on the basis of coronary height (CH) relative to the transcatheter valve leaflet height (TVH) of both the first and the second THV. When pertinent, each root type was further classified as either feasible or unfeasible for coronary re-access after redo TAVI based on THV commissural alignment relative to coronary ostia as described previously2 or valve-to-sinotubular-junction (VTSTJ) distance ≥2 mm.
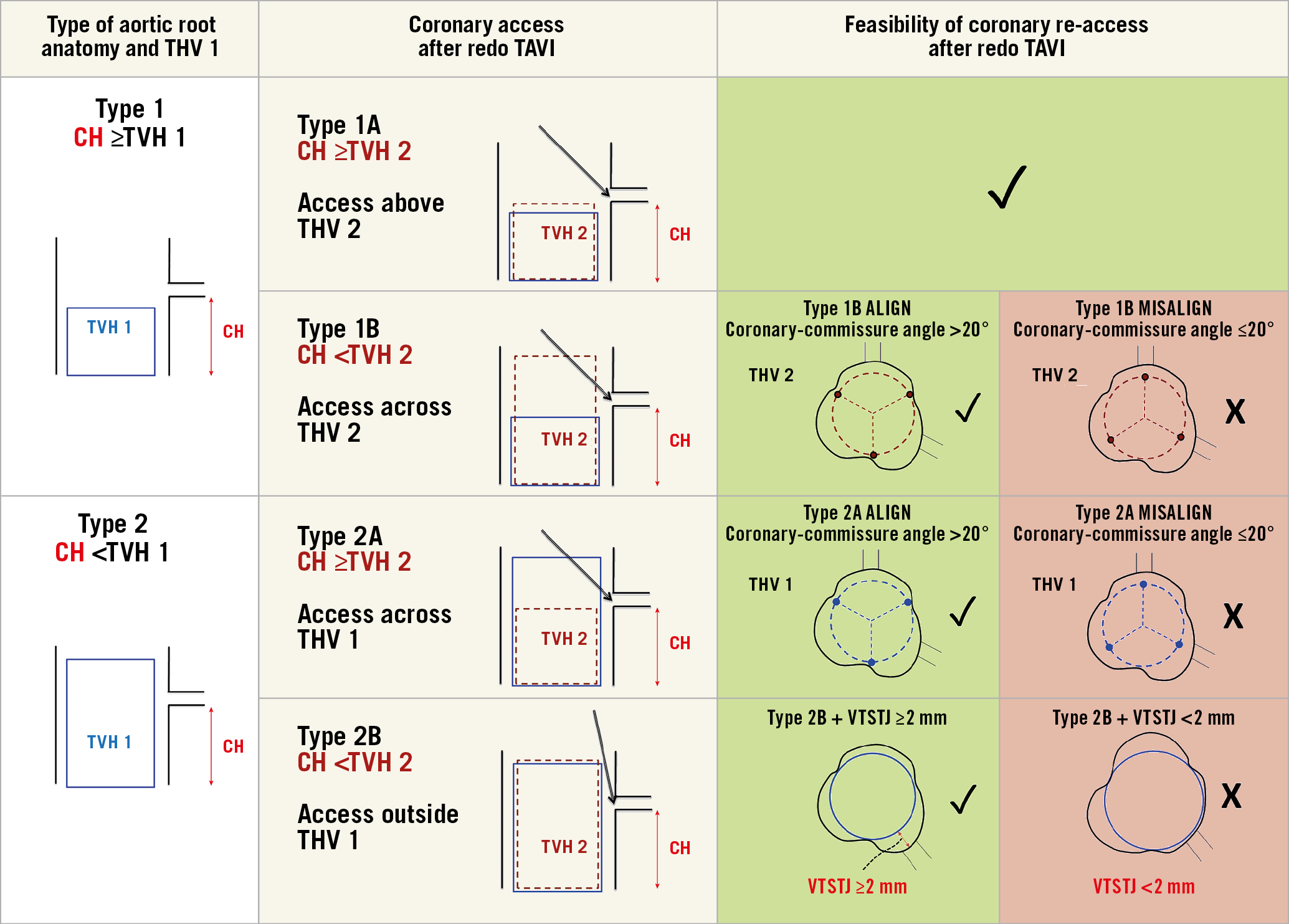
Figure 1. Proposed classification on the assessment of feasibility of coronary re-access after redo TAVI. Green panels suggest that coronary re-access would be highly feasible while red panels suggest that it would probably be unfeasible. CH: coronary height; THV: transcatheter heart valve; TVH: transcatheter valve leaflet height; VTSTJ: valve-to-sinotubular-junction height
As acknowledged by the authors, we need to recognise that this is purely an anatomic modelling study; it remains unknown whether there were differences in their predicted versus observed rates of unfeasible coronary access after redo TAVI. Reported coronary obstruction rates of 0.9% in the redo TAVI registry7 are significantly lower compared to the study’s predicted rates of 15% and 42% using VTC cut-offs of <2 mm and <4 mm, respectively. This may be explained by the three-dimensional nature of aortic root anatomy, which is not accounted for by the proposed VTC cut-offs. Although the investigators attempted to validate their findings in nine patients with multidetector computed tomography (MDCT) who underwent redo TAVI, none of them had a VTC <4 mm. Furthermore, no coronary re-access was attempted after the procedure, and no coronary obstruction was observed either. This remains a key limitation that prospective studies will need to address in order to refine technologies and techniques to overcome this problem.
The investigators should nonetheless be commended for their in-depth analysis of important structural relationships between coronary ostia and various THV platforms, and for highlighting an unaddressed problem facing redo TAVI. The contemporary nature of the study, potential of post-TAVI MDCT in identifying patients at risk for impaired coronary re-access and coronary obstruction, and implications of index THV design/selection on redo TAVI feasibility are strengths of the current study. As TAVI becomes the new standard of care in aortic valve replacement, there is a clear need to anticipate and mitigate challenges facing coronary access and redo TAVI – challenges that will need dedicated solutions from TAVI operators and device manufacturers alike.
Conflict of interest statement
G. Tang is a physician proctor with Medtronic and a consultant for Medtronic, Abbott Structural Heart and W.L. Gore & Associates. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

