
Introduction
Transcatheter heart valve (THV) degeneration will become increasingly common with the expansion of the indication for transcatheter aortic valve replacement (TAVR) to low-risk younger subjects with longer life expectancy1. Treatment of structural THV degeneration with the implantation of a second THV is feasible, but data on this subject are scant. In particular, there are concerns about the risk of coronary artery obstruction and the possibility of accessing the coronary ostia in case percutaneous coronary artery intervention should be needed in the future2,3,4.
It is crucial to understand the three-dimensional interaction among degenerated THV, coronary ostia and aortic root to guide preprocedural planning of TAVR-in-TAVR with the aim of minimising the risk of acute coronary artery occlusion and preserving future coronary access (CA). In fact, the implantation of a second THV will tilt up the leaflets of the previously implanted device, thereby creating a covered cylindric stent as tall as the commissural posts.
Methods
We defined the risk plane (RP) as the level under which the passage of a coronary catheter will be impossible after the second THV is implanted (Figure 1A). Based on 1) the relationship between the RP and the coronary ostia on computed tomography (CT), and 2) how CA is achieved with the first THV in place, we developed an algorithm to predict the risk of acute coronary occlusion and feasibility of future CA after TAVR-in-TAVR (Figure 1B).
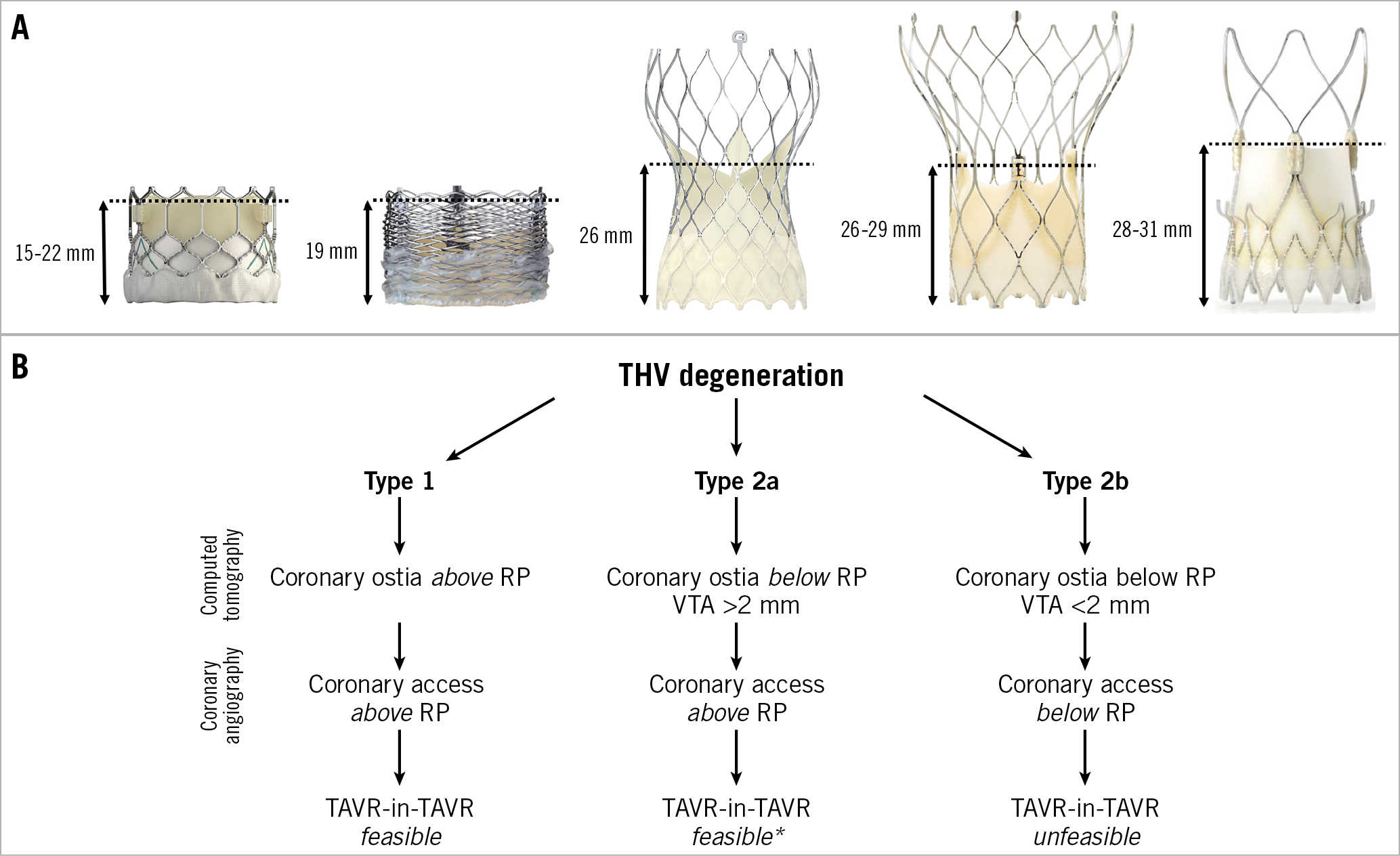
Figure 1. Risk planes of different THVs and proposed algorithm. A) Risk plane of SAPIEN 3, Lotus, Evolut R/PRO, Portico and ACURATE neo THVs. B) Preprocedural algorithm for evaluation of feasibility of CA after TAVR-in-TAVR. *CA challenging if TAVR-in-TAVR with two Evolut R/PRO or Portico THVs.
Results
According to aortic root anatomy, three different scenarios can be depicted with the SAPIEN XT/3 (Edwards Lifesciences, Irvine, CA, USA), CoreValve®/Evolut® (Medtronic, Minneapolis, MN, USA) (Figure 2) and other commercially available THVs (Supplementary Appendix 1, Supplementary Figure 1).
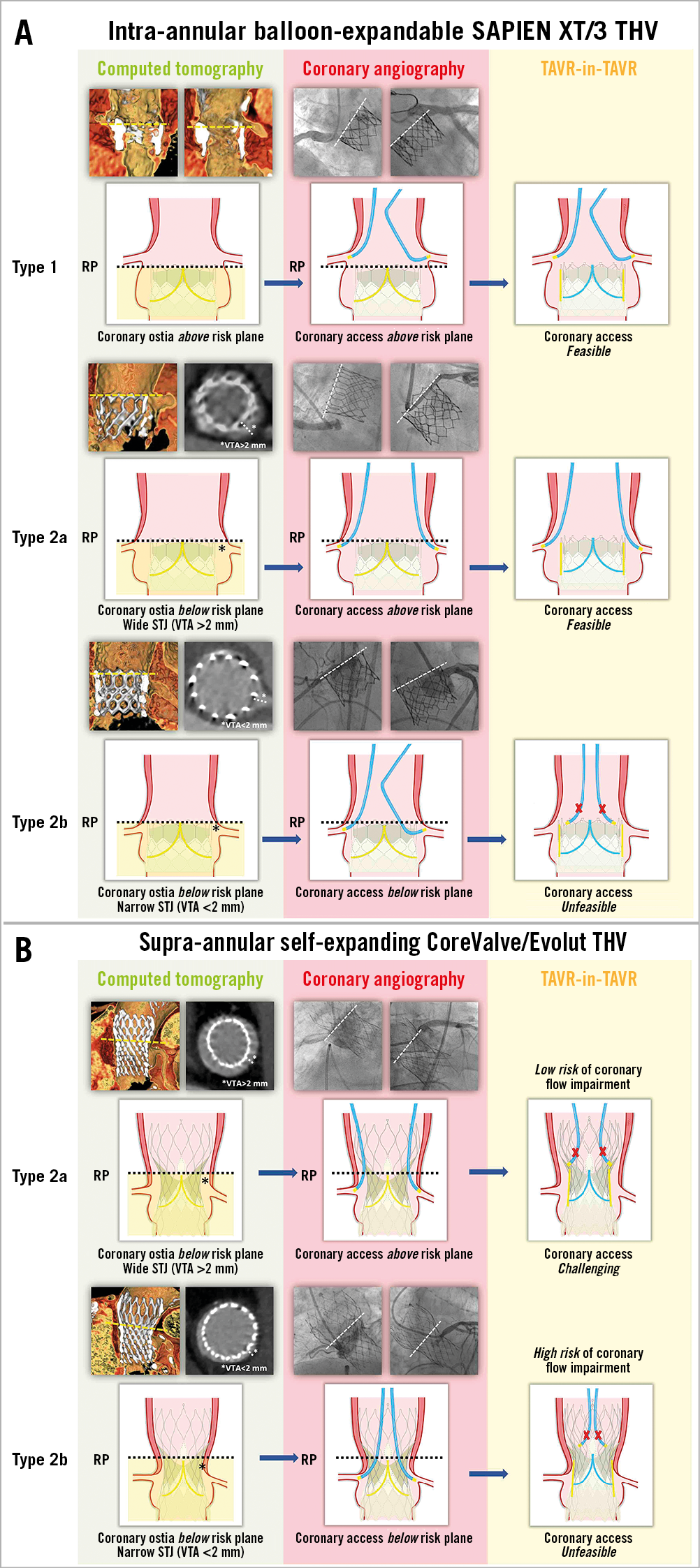
Figure 2. Different TAVR-in-TAVR scenarios with balloon-expandable SAPIEN XT/3 and self-expanding CoreValve/Evolut THVs.
A) TAVR-in-TAVR for intra-annular BE SAPIEN XT/3 degeneration. Type 1. As shown by CT imaging, the RP lies below the coronary ostia. Selective CA is achieved above the RP. After TAVR-in-TAVR, coronary cannulation is feasible above the valve frame. Type 2a. Coronary ostia are below the RP. The STJ is wide, with a computed tomography measured VTA >2 mm. Coronary cannulation is feasible from above the RP, outside the valve frame. In this case, CA after TAVR-in-TAVR should be possible. Type 2b. Coronary ostia are below the RP. The STJ is narrow, with a VTA <2 mm. Coronary cannulation is feasible just through one of the upper row cells. After TAVR-in-TAVR, CA will be impossible, with a high risk of acute coronary flow impairment. B) TAVR-in-TAVR for supra-annular SE CoreValve/Evolut degeneration. Given the high position of valve leaflets, coronary ostia will almost always be under the RP (Type 2). Type 2a. The STJ is wide, with a VTA >2 mm. CA is obtained through a cell above the RP. TAVR-in-TAVR with a second SE device will not cause coronary flow impairment, but the two layers of overlapping struts will make future CA challenging, if not impossible. Type 2b. The STJ is narrow, with a VTA <2 mm. CA is gained from below the RP. TAVR-in-TAVR carries a high risk of acute coronary artery obstruction.
Discussion
DEGENERATION OF THE INTRA-ANNULAR BALLOON-EXPANDABLE SAPIEN XT/3 THV
The balloon-expandable (BE) prostheses SAPIEN XT and SAPIEN 3 have an intra-annular design, a low frame profile (frame height 14-19 mm for the SAPIEN XT, 15.5-22.5 mm for the SAPIEN 3) and an upper row of open cells (with a diameter of 4.4-6.8 mm for the SAPIEN 3, 40% smaller for the SAPIEN XT). Commissural tabs and leaflet attachment are located in three of the 12 open cells. Therefore, the RP is approximately 1 mm below the upper margin of the valve frame (Figure 2A).
CORONARY OSTIA ABOVE THE RISK PLANE (Type 1)
When the RP lies below the coronary ostia, because of either a high coronary origin or a low THV implantation, CA can be gained from above the RP. In this case, TAVR-in-TAVR with a second intra-annular THV (avoiding high implantation) will not impede subsequent coronary cannulation. Implantation of a self-expanding (SE) supra-annular Medtronic Evolut R/PRO inside the first THV is also possible.
CORONARY OSTIA BELOW THE RISK PLANE (Type 2)
When the coronary ostia are low or the first THV is implanted high, the origin of one or both coronaries will be below the RP. In this situation, CT analysis is important to assess the valve-to-aorta distance (VTA), defined as the distance between the prosthesis frame at the level of the RP and the aortic wall. A free space of >2 mm is necessary to navigate a 6 Fr catheter behind the prosthesis struts and engage the coronary ostium5. Coronary angiography after the index TAVR will single out two different scenarios.
CORONARY ACCESS FROM ABOVE THE RISK PLANE (Type 2a)
In patients with a wide sinotubular junction (STJ) and a CT-assessed VTA >2 mm, CA from above the RP outside the valve frame needs to be confirmed by selective coronary angiography. In this case, TAVR-in-TAVR with a second BE device is feasible, but excessive oversizing and post-dilation should be avoided in order not to flare the superior part of the THV and to preserve the VTA. The use of an SE device with taller commissural posts for TAVR-in-TAVR is also possible, but coronary cannulation might be more challenging because of the possibility of placing a neo-commissure in front of the coronary ostia.
Coronary access from below the risk plane (Type 2b)
If the STJ is narrow and VTA is <2 mm, coronary cannulation will be possible only through the upper row cells of the THV frame. Since the leaflets of the first BE THV will form a cylinder when tilted up by the new BE or SE THV, CA after TAVR-in-TAVR will be unfeasible, with the risk of coronary occlusion. According to a recent study based on aortic angiography after TAVR, this unfavourable anatomical scenario could be found in up to 20% of patients treated with a SAPIEN 3 THV5. However, considering that coronary cannulation was not attempted, we cannot exclude overestimation or underestimation of this situation.
DEGENERATION OF SUPRA-ANNULAR SELF-EXPANDING COREVALVE/EVOLUT THV
The SE CoreValve, Evolut R/PRO have a supra-annular design, a taller frame (45-55 mm) and a commissure height of 26 mm. The risk of coronary obstruction with this type of prosthesis is minimised by their narrow waist and the possibility of recapture after partial deployment in case of coronary flow impairment. To cannulate the coronary ostia, a catheter needs to pass through one of the diamond-shaped cells of the stent frame. Given the commissural post height, the RP of supra-annular Evolut R/PRO devices will be considerably higher compared to BE intra-annular THVs (Figure 2B).
CORONARY OSTIA ABOVE THE RISK PLANE (Type 1)
This situation is extremely uncommon with correctly implanted supra-annular devices. In fact, Evolut R/PRO THVs have 26 mm high commissures that invariably expand above the coronary ostia. Accordingly, the RP is almost always above the coronaries, and CA from above the RP is feasible just in anecdotal cases with very high coronary origin or inappropriately low THV implantation. Even though TAVR-in-TAVR with a second Evolut R/PRO device is feasible, selective coronary engagement will be very challenging or impossible. In fact, given the impossibility of orientating the THV precisely during implantation, the two stent layers above the RP might overlap, leaving insufficient room to permit the passage of a coronary catheter. An exception to this might be the situation of an extremely large aortic root allowing the passage of the catheter outside the valve frame between the prosthesis and the aortic wall. Of note, TAVR-in-TAVR with a low implanted lower-frame intra-annular BE device might not achieve complete vertical displacement of the degenerated leaflets of the first supra-annular SE device.
CORONARY OSTIA BELOW THE RISK PLANE (Type 2)
This scenario is by far the most common with supra-annular SE devices.
Coronary access from above the risk plane (Type 2a)
CA from above the RP, although potentially challenging, is feasible only if the VTA is >2 mm. In this situation, TAVR-in-TAVR is possible with the same caveats as in the aforementioned Type 1 scenario.
Coronary access from below the risk plane (Type 2b)
In patients with a narrow STJ, the VTA at the RP might be <2 mm. Accordingly, coronary cannulation is possible just through a cell located below the RP. When the leaflets of the first SE THV are tilted up after the implantation of the second prosthesis, there will be a 26 mm tall barrier in front of the coronary ostia. Subsequent CA will be impossible and TAVR-in-TAVR will probably cause coronary artery obstruction, regardless of the type of prosthesis used.
Limitations
The main limitation of this short report is the lack of data. Further studies are needed to assess the prevalence of the described scenarios in a contemporary TAVR population and therefore to predict the percentage of patients potentially unsuitable for TAVR-in-TAVR.
Conclusion
Percutaneous treatment of a failing THV poses challenges in terms of preventing acute coronary obstruction and preserving future CA. Unlike TAVR in surgical aortic valves, novel leaflet splitting techniques such as BASILICA may be less effective when THV neo-commissures are not aligned to those of the native aortic valve. Computed tomography and coronary angiography are important and complementary for correct preprocedural planning of the procedure. CA after TAVR-in-TAVR will be feasible with type 1 (coronary ostia and CA above the RP) and type 2a scenarios (coronary ostia below the RP, VTA >2 mm, CA above the RP). On the other hand, in patients with type 2b (coronary ostia and CA below the RP, VTA <2 mm) TAVR-in-TAVR will carry a high risk of coronary artery obstruction. Given their different design and their higher risk plane, supra-annular devices are more likely to impede CA after TAVR-in-TAVR. Notably, redo TAVR with the use of partially or fully repositionable devices might reduce the risk of irreversible coronary obstruction in high-risk cases.
|
Impact on daily practice Although hypothesis-generating, the aspects described in this short report should be considered when selecting the THV to implant in younger patients with longer life expectancy. |
Conflict of interest statement
G. Tarantini reports honoraria for lectures/consulting from Medtronic, Edwards, Boston Scientific, GADA and Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

