Abstract
Background: It is unknown whether there are differences in coronary access after transcatheter aortic valve replacement (TAVR) between bicuspid and tricuspid anatomy.
Aims: Our aim was to investigate coronary access after TAVR using a self-expanding transcatheter heart valve (THV) in bicuspid versus tricuspid aortic valves (BAV vs TAV), based on CT simulation.
Methods: A total of 86 type 0 BAV, 70 type 1 BAV, and 132 TAV patients were included. If the coronary ostium faced the sealed parts of the THV or the tilted-up native leaflet (NL), this was defined as THV- or NL-related challenging coronary access, respectively. If coaxial engagement was not allowed due to interference from the unwrapped frame, THV-related complex coronary access was defined.
Results: The incidence of THV-related challenging coronary access was 21.2% for the left coronary artery (LCA) and 17.7% for the right coronary artery (RCA), and type 0 BAV patients encountered fewer THV-related challenging LCA access than their TAV counterparts (OR 0.42, 95% CI: 0.20-0.89). NL-related challenging coronary access was observed in 3.1% for LCA and 1.4% for RCA, and THV-related complex coronary access was identified in 5.9% for LCA and 17.0% for RCA; however, no significant differences were found among groups. The proportion of optimal fluoroscopic viewing angles suitable for guiding LCA engagement was similar among groups (64.0% vs 70.0% vs 62.1%), but those suitable for guiding RCA engagement were significantly higher in the type 0 BAV group (31.4% vs 4.3% vs 9.1%).
Conclusions: Coronary access may be challenging or complex in a significant proportion of both BAV and TAV patients after TAVR. Type 0 BAV anatomy may be more favourable for post-TAVR coronary access.
Introduction
Transcatheter aortic valve replacement (TAVR) has revolutionised the treatment of severe aortic stenosis and is equal or superior to surgical aortic valve replacement across the entire spectrum of surgical risk1234. As TAVR indications are now expanding into lower-risk and younger patients who have a longer life expectancy, there will be an increasing need for coronary angiography or intervention after TAVR. These patients endure a high cumulative risk of progressive/unstable coronary artery disease which shares similar pathogenic or aggravating factors with aortic stenosis56. Coronary access issues may be one of the major concerns of long-term management after TAVR, because the geometric interaction between the coronary ostia and the transcatheter heart valves (THVs), as well as the tilted-up native aortic valve leaflets, may lead to difficulties in obtaining access to the coronary ostia78.
Recently, several studies utilised computed tomography (CT) simulation analysis to evaluate the feasibility of coronary access after TAVR using self-expanding or balloon-expandable THVs in tricuspid aortic valve (TAV) patients. These studies indicated coronary access might be challenging in a significant proportion of the non-selected patients and explained the main mechanism of potential interferences91011. However, patients with bicuspid aortic valve (BAV) were excluded from these studies, although BAV is more common than TAV in the younger aortic valve stenosis population (e.g., from 27.9% in those older than 80 years to 62.5% in those 61 to 70 years old) and the prevalence of BAV in TAVR patients is expected to considerably increase as TAVR indication expands1213. Despite several theoretical perspectives, it remains unknown whether the differences between BAV versus TAV in aortic root features and interactions with THVs impact the difficulties of coronary access after TAVR14.
This study aimed to investigate coronary access after TAVR using a self-expanding THV in patients with BAV versus TAV, based on CT simulation.
Methods
Study population
Between January 2014 and December 2019, consecutive patients who underwent TAVR for severe aortic stenosis using a single self-expanding VenusA-Valve (Venus Medtech) at the Department of Cardiology, West China Hospital, were screened for inclusion. Patients who did not undergo electrocardiographically-gated contrast-enhanced CT after TAVR, or whose CT image quality was inadequate, were excluded from the analysis. TAVR procedures were performed according to standard techniques, without intentionally performing commissural alignment. The study was approved by the Institutional Review Board, and written informed consent was obtained from all patients.
CT image acquisition
CT image acquisition protocols are outlined in Supplementary Appendix 1. All images were transferred to the FluoroCT software (version 3.2, Circle Cardiovascular Imaging) for analysis.
Pre-TAVR CT analysis
Pre-TAVR CT analysis was performed according to current guidelines15. Based on the number of commissures (2 or 3) and the presence or absence of a raphe, we classified aortic valve morphology as follows: type 0 BAV, type 1 BAV, and TAV16.
Design features of VenusA-Valve
The design features of the VenusA-Valve relevant to coronary access after TAVR are shown in Supplementary Figure 1. The dimensions were measured using the manufacturer's project files (Supplementary Table 1). Briefly, its design is similar to that of CoreValve/Evolut (Medtronic) THVs, which are the world's most widely used self-expanding THVs. The frame is made of a network of diamond shapes consisting of alternating cells and nodes. It is wrapped with a bioprosthetic skirt between inflow and leaflet nadir levels, and hosts three bioprosthetic valve commissural posts, comprising a commissural triangle and a commissural diamond, which are also wrapped in bioprosthetic material.
Post-TAVR CT analysis and definition
Key post-TAVR CT measurements are summarised in Figure 1A-Figure 1I. The distances from the left or right coronary artery (LCA/RCA) ostium to the THV inflow plane and the THV short axis were defined as ostium-inflow distance and ostium-THV distance, respectively. The ostium-commissure angle was measured between the projection of the coronary ostium midpoint onto the THV commissure plane along the long axis and its closest THV commissure. The perpendicular distance from the nadir of the left or right coronary sinus (LCS/RCS) to the THV inflow plane was measured as the THV implantation depth.
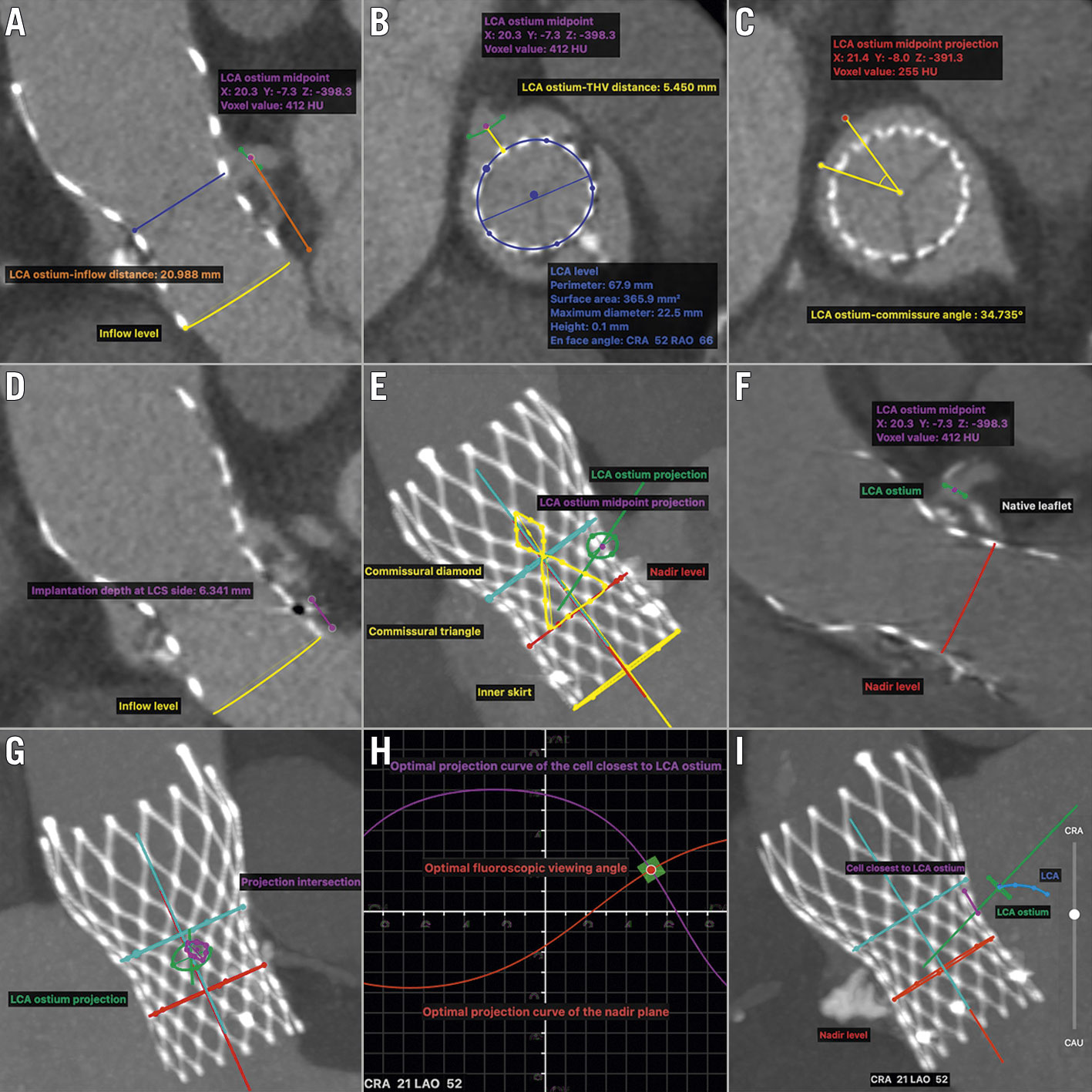
Figure 1. Post-TAVR CT measurements of the geometric interaction between deployed THV and coronary ostia. A-D. The measurements of LCA ostium-inflow distance, LCA ostium-THV distance, LCA ostium-commissure angle, and implantation depth at LCS side, respectively. E. MIP en face view of the commissural post (yellow triangle and diamond) closest to the LCA ostium. If the ostium faced the commissural post or inner skirt, THV-related challenging LCA access was identified. F. Double-oblique long-axis plane of the THV cutting through its centreline and LCA ostium. If the ostium faced the tilted-up bulky native leaflet, NL-related challenging LCA access was identified. G. MIP en face view of LCA ostium projection onto THV long-axis plane along the direction between LCA ostium midpoint and the centre of THV short axis. If the short-axis diameter of the intersection of LCA ostium projection and the closet cell projection was less than 2 mm (cut-off value was chosen based on 6 Fr catheter size), a coaxial catheter engagement was not allowed, and THV-related complex LCA access was identified. H-I. Double-S curve method was used to calculate the optimal fluoroscopic viewing angle for LCA access, in which view the THV nadir plane and the cell closest to LCA ostium were both in plane and the ostium was nearly in plane while the THV was fully elongated. CT measurements of the geometric interaction between deployed THV and RCA ostium could be done using the same methods. CRA: cranial; CT: computed tomography; LAO: left anterior oblique; LCA: left coronary artery; LCS: left coronary sinus; MIP: maximum intensity projection; NL: native leaflet; RAO: right anterior oblique; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve
Four coronary access classifications were defined according to the interferences and the corresponding theoretical severity (Figure 1E-Figure 1G, Central illustration A). If the coronary ostium faced the sealed commissural post or inner skirt, these structures would significantly interfere with coronary engagement. We defined this as “THV-related challenging coronary access”. If the coronary ostium faced the tilted-up native leaflets (NL), this would also significantly interfere with coronary engagement, and we defined this as “NL-related challenging coronary access”. For non-challenging coronary access, coronary engagement may also be interfered with by the THV frame not being wrapped with bioprosthetic material. If a coaxial coronary engagement was not allowed, this was defined as “THV-related complex coronary access”. Other coronary access was classified as “favourable coronary access”.
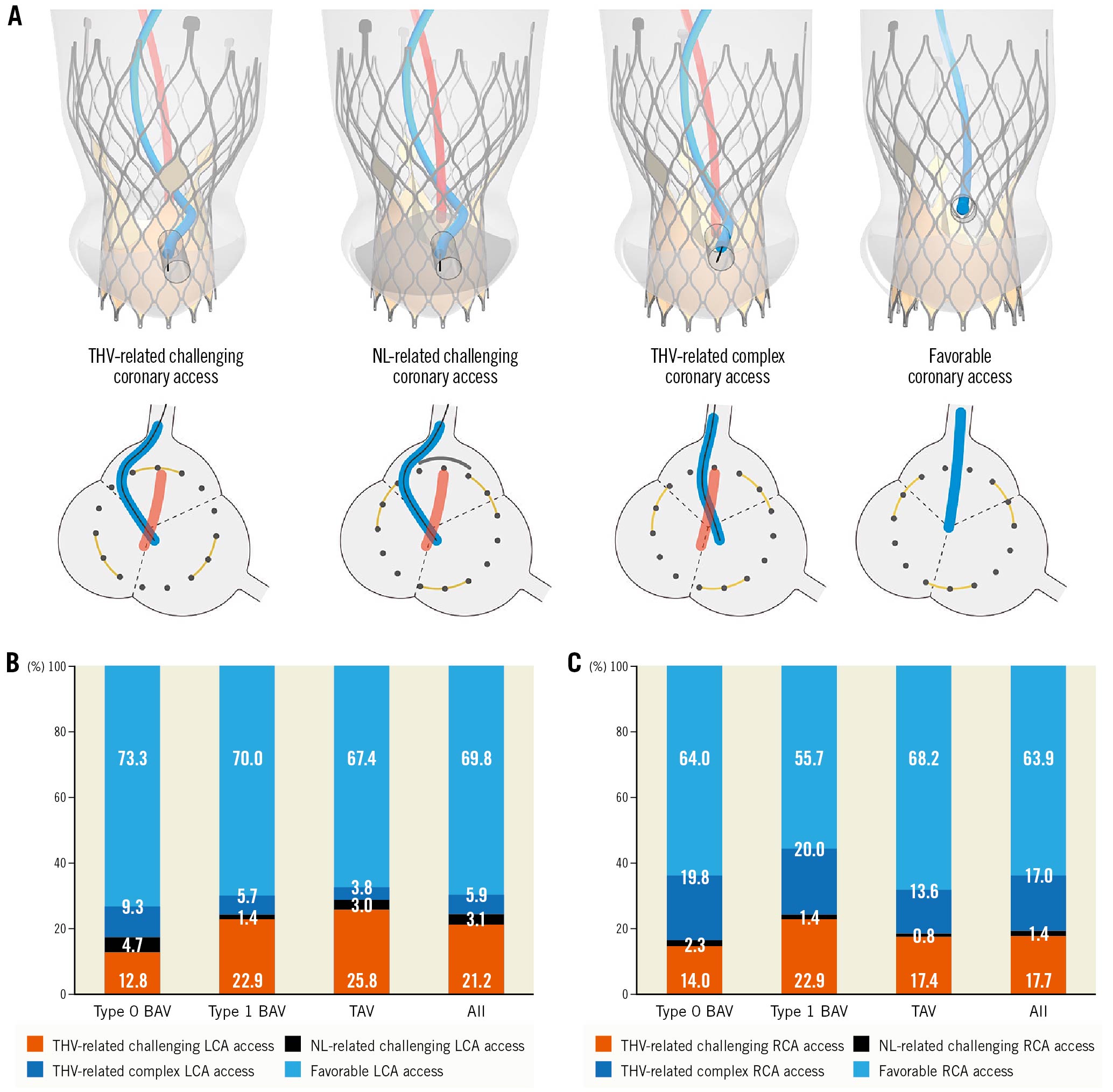
Central illustration. Coronary access after TAVR in bicuspid versus tricuspid aortic stenosis. A) A graphic representation of coronary access classification examples. The yellow arcs, grey arcs, and dark grey dots indicated the cross section of the sealed commissural post, tilted-up native leaflet, and THV metallic frame, respectively. B & C) Stacked bars with the distribution of CT-identified coronary access classification stratified by native aortic valve morphology, respectively. BAV: bicuspid aortic valve; LCA: left coronary artery; NL: native (aortic valve) leaflet; RCA: right coronary artery; TAV: tricuspid aortic valve; THV: transcatheter heart valve
We used the double S-curve method to calculate the optimal fluoroscopic viewing angle, in which the THV nadir plane and the closest cell to the coronary ostium were both in plane. The coronary ostium was also nearly in plane while the THV was fully elongated, allowing the optimal identification of the closest cell and clear identification of the coronary ostium (Figure 1H, Figure 1I). However, the closest cell of challenging coronary access was wrapped in bioprosthetic material or covered by a native leaflet, and this method was not applicable. Meanwhile, due to the physical limitations of current angiography systems, extreme angulations, although optimal, may not be practical. A practical projection range described by a previous study was adopted17.
Follow-up
During follow-up, the indication for coronary angiography or intervention was judged by a consultant cardiologist. In patients who underwent coronary access, coronary engagement was defined as “unsuccessful” when it was deemed impossible to obtain selective or semi-selective cannulation.
Statistical analysis
Continuous variables are presented as means±standard deviation or median (interquartile range [IQR]) and were compared using One-way Analysis of Variance or Kruskal–Wallis H tests, respectively. Categorical variables are presented as counts and percentages and were compared using the chi-square test. Bonferroni correction was utilised for post hoc multiple comparisons. Paired categorical data were analysed using McNemar's test. The cumulative incidence of time-to-event outcomes was estimated using the Kaplan-Meier method. Multivariable logistic regression analysis was utilised to determine the predictors of THV-related challenging coronary access. Odds ratios (ORs) were reported with their 95% confidence intervals (CIs). Discrimination of the final model was assessed using the C-statistic. The relative importance of each predictor was measured using the partial chi-square statistic minus the predictor degree of freedom (df). A two-sided p-value of less than 0.05 was considered statistically significant. All analyses were performed using Stata 15.1 SE (StataCorp).
Results
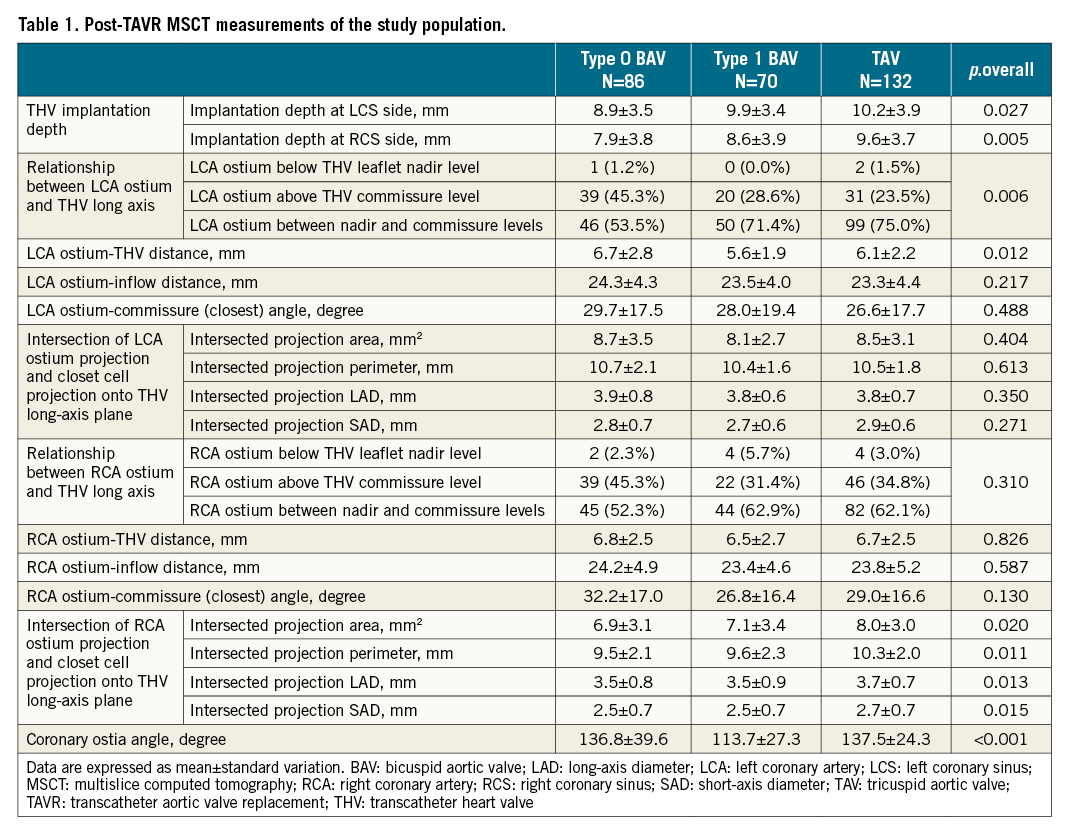
A total of 86 type 0 BAV, 70 type 1 BAV, and 132 TAV patients were included in this study. Baseline characteristics, TAVR procedural characteristics, and in-hospital outcomes are outlined in Supplementary Table 2. The mean age was 73.7±7.0 years, females accounted for 46.2%, the average STS-PROM score was 6.4±4.4%, and 24.7% of patients had pre-existing coronary artery disease. Most pre-TAVR CT measurements were significantly different among groups. Particularly, LCA ostium was significantly higher in the type 0 BAV group than in the type 1 BAV or TAV group (15.4±3.7 mm vs 13.2±3.7 mm or 12.9±2.5 mm, all p<0.001); however, the difference was not observed in RCA ostium height (15.8±3.5 mm vs 14.8±3.8 mm vs 15.5±3.3 mm, p.overall=0.173).
The geometric interaction between deployed THV and coronary ostia
Post-TAVR CT measurements are summarised in Table 1. In BAV, THVs are more likely to be implanted shallowly, especially in the type 0 BAV group (LCS side: 8.9±3.5 vs 9.9±3.4 vs 10.2±3.9 mm, p.overall=0.027; RCS side: 7.9±3.8 vs 8.6±3.9 vs 9.6±3.7 mm, p.overall=0.005). Along the THV long axis, most LCA ostia were located between the nadir and commissure levels (67.1%), while 1.0% of patients had LCA ostium below the nadir level facing the skirt, and the corresponding percentages for RCA ostium were 59.4% and 3.5%, respectively. Along the THV circumference, coronary ostia were randomly distributed without a significant difference among groups (Supplementary Figure 2).
CT-identified coronary access classification
The distribution patterns of CT-identified coronary access classification are outlined in Central illustration (B, C).
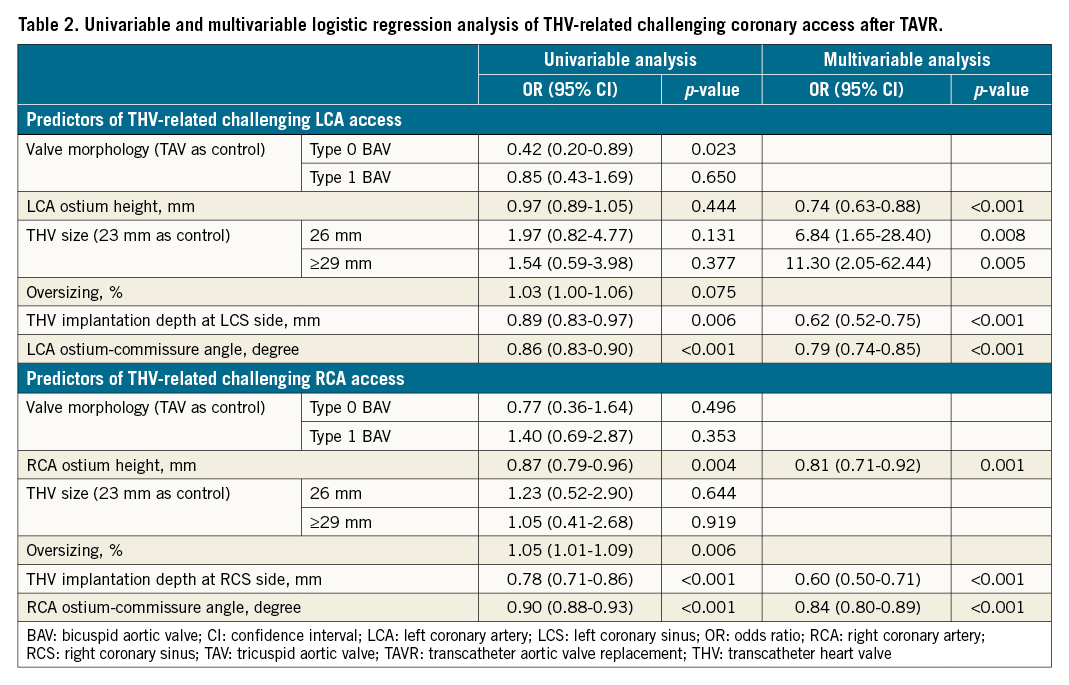
The incidence of THV-related challenging LCA access was 21.2%, in which 17.7%, 2.4%, and 1.0% were interfered with by commissural triangles, commissural diamonds, and skirts, respectively. The corresponding percentages for THV-related challenging RCA access were 17.7%, 12.2%, 2.1%, and 3.5%, respectively. Compared with the TAV group, the incidence of THV-related challenging LCA access was significantly lower in the type 0 BAV group (12.8% vs 25.8%; OR 0.42, 95% CI: 0.20-0.89), but was similar in the type 1 BAV group (22.9% vs 25.8%; OR 0.85, 95% CI: 0.43-1.69) (Figure 2). No significant difference was found among groups regarding the incidence of THV-related challenging RCA access (14.0% vs 22.9% vs 17.4%). On multivariable analysis, ostium-commissure angle, implantation depth, coronary ostium height and THV size (only for LCA) were found to be the independent predictors of THV-related challenging coronary access (Table 2). The final models had excellent discrimination (C-statistic: 0.96 and 0.94; respectively), and the ostium-commissure angle (X2-df : 44.94 and 41.71, respectively) contributed most to their performance.
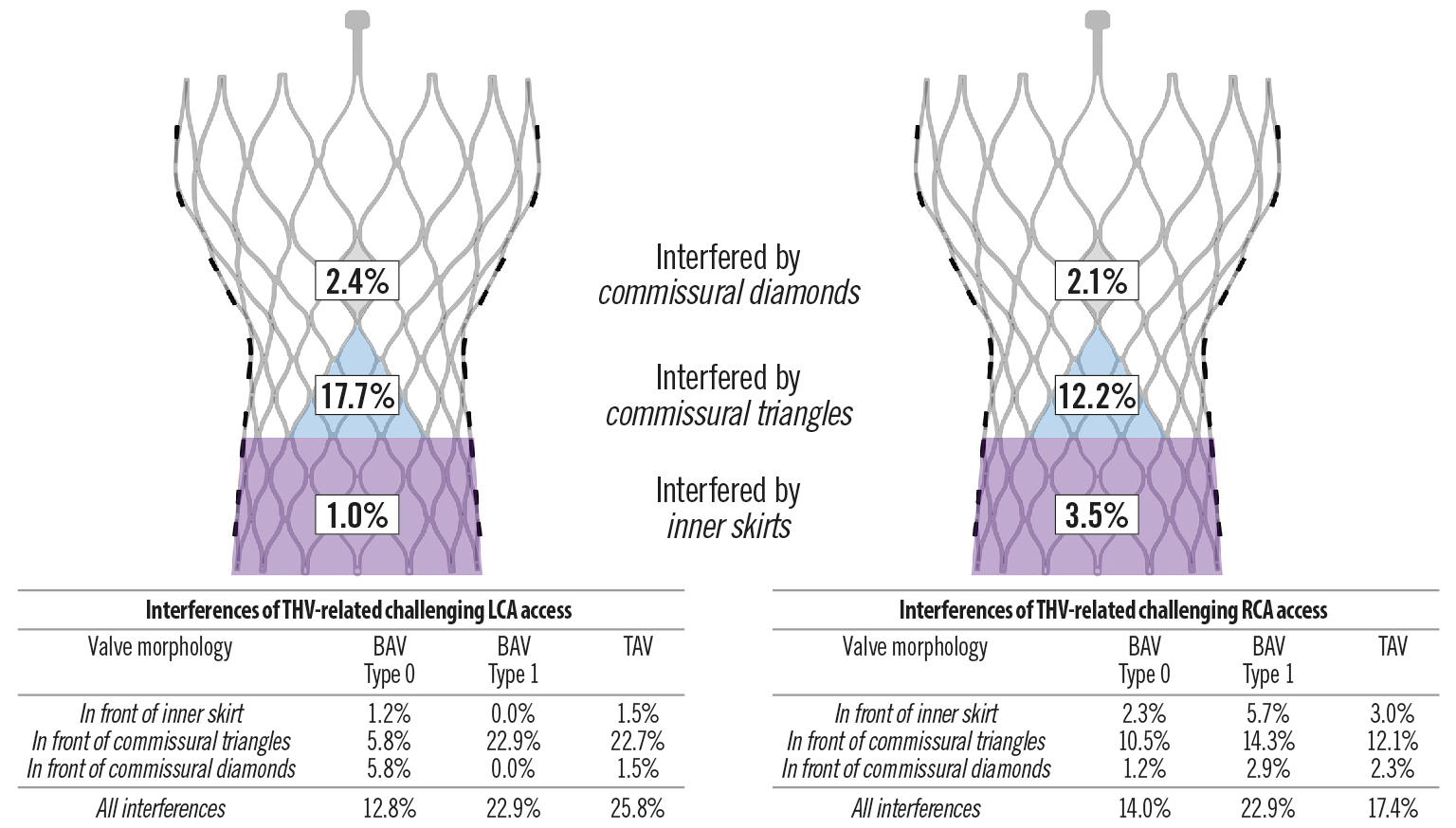
Figure 2. Features of THV-related challenging coronary access according to post-TAVR CT analysis. BAV: bicuspid aortic valve; LCA: left coronary artery; RCA: right coronary artery; TAV: tricuspid aortic valve; THV: transcatheter heart valve
The incidence of NL-related challenging LCA access was 3.1%, and one patient with the LCA ostium also facing the skirt was not counted. The corresponding percentage for RCA access was 1.4%, and two patients with the RCA ostium also facing the skirt or commissural triangle were not counted. There were no significant differences among groups (4.7% vs 1.4% vs 3.0%, p.overall=0.514; 2.3% vs 1.4% vs 0.8%, p.overall=0.626; respectively).
THV-related complex LCA access was observed in 5.9% of patients, and the percentage in the BAV groups was numerically higher than in the TAV group (9.3% vs 5.7% vs 3.8%, p.overall=0.240). The incidence of THV-related complex RCA access was 17.0%, and no significant difference was found among groups (19.8% vs 20.0% vs 13.6%, p.overall=0.373). Particularly, the incidence of THV-related complex LCA access was significantly lower than that of corresponding RCA access (5.9% vs 17.0%, p<0.001).
Association between CT-identified coronary access classification and unsuccessful coronary engagement
The estimated 5-year cumulative incidence of indicated coronary access was 15.8% (95% CI: 7.3%-32.0%). After a median follow-up of 19.4 months, 17 coronary engagements in 9 patients were performed (9 for LCA, and 8 for RCA). The procedure details are presented in Supplementary Table 3. At engagement-level analysis, unsuccessful rates were strongly associated with CT-identified coronary access classification (THV-related challenging vs THV-related complex vs favourable coronary access: 80.0% [4/5] vs 33.3% [1/3] vs 0.0% [0/9], p.overall=0.004), and the rate of selective engagement was numerically higher in favourable LCA access than in favourable RCA access (80.0% [4/5] vs 25.0% [1/4], p=0.206).
Optimal fluoroscopic viewing angles for coronary access after TAVR
Optimal fluoroscopic viewing angles for coronary access after TAVR are represented in Figure 3. The median optimal viewing angle was LAO (left anterior oblique) 26.1°/CRA (cranial) 14.3° (IQR: LAO 16.2°-39.5°/CRA 2.9°-25.2°) for LCA access and LAO 55.5°/CRA 35.5° (IQR: LAO 30.9°-80.0°/CRA 19.3°-41.6°) for RCA access, and the percentage of the angles within the practical range was 87.2% and 18.1%, respectively. There were significant differences in the optimal viewing angles among groups. The percentages of optimal viewing angles suitable for guiding LCA engagement were similar among groups (64.0% vs 70.0% vs 62.1%, p.overall=0.532), but those suitable for guiding RCA engagement were significantly higher in the type 0 BAV group than in the other groups (31.4% vs 4.3% vs 9.1%, p.overall<0.001) (Supplementary Table 4).
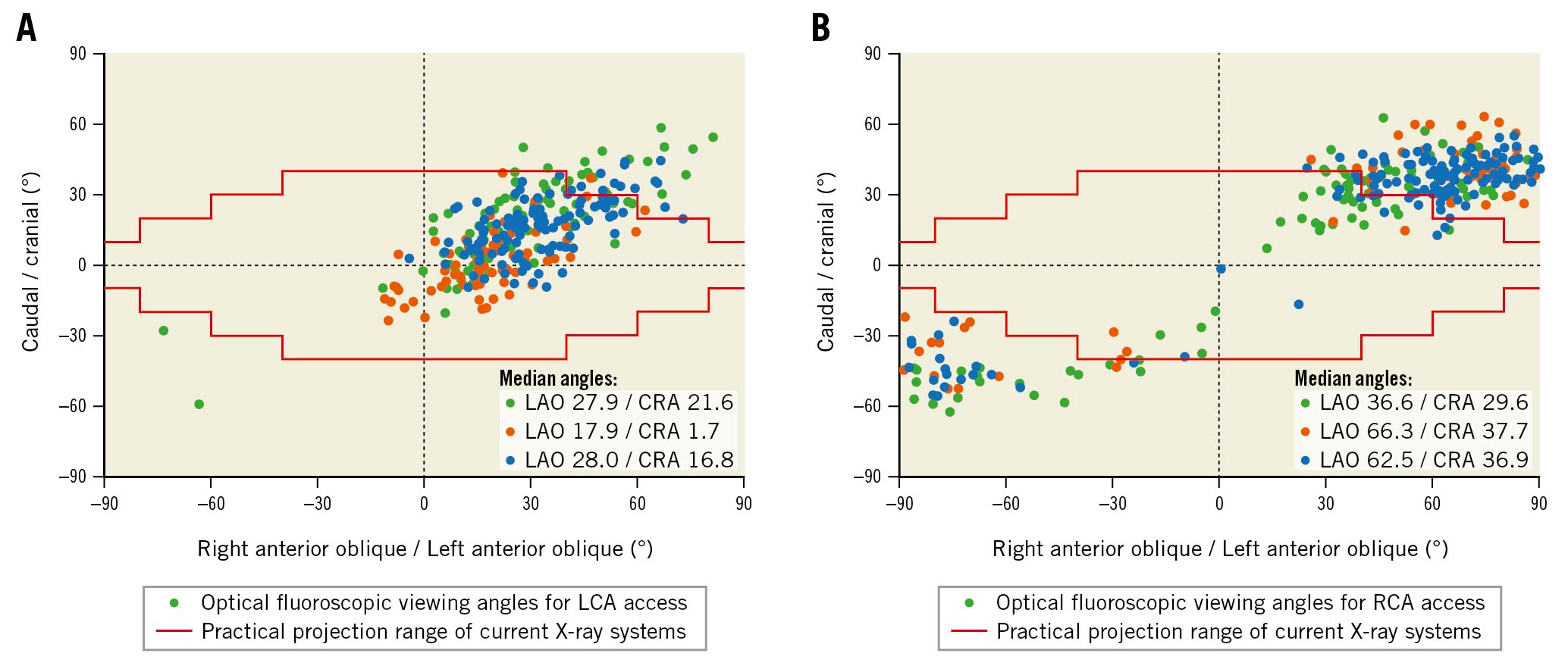
Figure 3. Optimal fluoroscopic viewing angles for coronary access after TAVR. A, B) Scatter plots representing CT-identified individual patients’ optimal fluoroscopic viewing angles for LCA and RCA access, respectively. The forest green, dark orange, and navy solid circles represent the optimal angles of patients with type 0 BAV, type 1 BAV, and TAV, respectively. The red stepped lines highlight the practical projection range of current X-ray systems. The angles between the two red stepped lines are practical. BAV: bicuspid aortic valve; LCA: left coronary artery; RCA: right coronary artery; TAV: tricuspid aortic valve; TAVR: transcatheter aortic valve replacement
Discussion
To our knowledge, this is the first study to investigate coronary access after TAVR in BAV versus TAV. The main findings of this study are as follows: 1) the incidence of THV-related challenging coronary access was 21.2% for LCA and 17.7% for RCA, and type 0 BAV patients encountered fewer THV-related challenging LCA access than their TAV counterparts (OR 0.42, 95% CI 0.20 -0.89); 2) NL-related challenging coronary access was observed in 3.1% for LCA and 1.4% for RCA, and THV-related complex coronary access was identified in 5.9% for LCA and 17.0% for RCA; however, no significant differences were found among groups; 3) the proportion of theoretical optimal fluoroscopic viewing angles that can be used to guide LCA engagement were similar among groups (64.0% vs 70.0% vs 62.1%), but those that can be used to guide RCA engagement (31.4% vs 4.3% vs 9.1%) were significantly higher in the type 0 BAV group.
Recently, several CT studies showed that the sealed commissural post and skirt were the main mechanisms of potential interference for self-expanding THVs in coronary access after TAVR and resulted in a considerable proportion of challenging coronary access; however, only TAV patients were investigated91011. As the prevalence of BAV in TAVR patients is expected to increase considerably, it is also essential to elucidate the geometric interaction between the coronary ostia and the THVs in this population. Our study confirmed the findings of previous studies in both BAV and TAV patients. We further explored the independent predictors for THV-related challenging coronary access: the angle between the coronary ostium and THV commissure, THV implantation depth, coronary ostium height, and THV sizes. However, the incidence of challenging THV-related coronary access in our TAV group was lower than that reported by previous studies, except one using a very rigorous CT definition (e.g., 25.8% vs 34.8% for LCA access and 17.4% vs 25.8% for RCA access compared with the RESOLVE study). This inconsistency may be attributable to the higher THV implantation in previous studies using the repositionable Evolut system, which could achieve the optimal THV position. Interestingly, we found that type 0 BAV patients encountered fewer THV-related challenging LCA access than TAV patients (12.8% vs 25.8%), which may be explained by their anatomical feature (significantly higher LCA ostium), although there was unfavourable interaction with the THV for coronary access (higher THV implantation). These findings emphasise that the geometric interaction between the coronary ostia and the THV is influenced by the anatomical features of the aortic root.
Theoretically, the displaced bulky native leaflets may impede coronary access even if the sealed part of the THV does not interfere with the path to the coronary ostia. However, previous studies did not factor these leaflets into the prediction of the difficulty of coronary access after TAVR. This may be due to the difficulty in assessing their location and bulkiness due to their close proximity to the metallic THV frame whose blooming artefact was remarkable on CT images9101118. In this study, we found that NL-related challenging coronary access was identified in a small number of patients. A possible explanation for this discrepancy may be that the calcium burden and/or the thickness of native leaflets in our patients were much higher than in previous studies. No significant differences were observed among BAV and TAV groups regarding NL-related challenging coronary access; nevertheless, unidentified cases may also exist in this study, and the result should be interpreted with caution. Additionally, we evaluated the impact of the unwrapped metallic frame on non-challenging coronary access using a quantification method. One previous study reported that the closest vertically-aligned cell could allow selective access to the coronary ostia in non-challenging coronary access, although the cell located below or above the ostial plane may increase the difficulty9. However, the high rate of semi-selective coronary engagement after TAVR in the real world (12.0% - 31.7% in the RE-ACCESS study) may challenge the abovementioned viewpoint8. Our study showed that THV-related complex coronary access, which may significantly reduce the possibility of selective engagement, was common, especially for RCA. This may be attributable to the smaller dimensions of the RCA ostium and was consistent with the greater difficulty of RCA engagement in previous studies819. Nevertheless, there was no significant difference among the BAV and TAV groups, which indicated that this type of interference might not be associated with aortic valve morphology subtypes.
The performance of the proposed CT-identified coronary access classification for the prediction of coronary engagement difficulty was initially validated in our study, signalling that a significant proportion of patients would encounter coronary access issues after TAVR. In patients with a longer life expectancy, it may be reasonable to simplify future coronary access during the first TAVR. Theoretically, lower THV implantation can reduce the riskof THV-related challenging coronary access, but it is not an ideal option because of the increased risk of pacemaker implantation and paravalvular regurgitation, which adversely affect outcomes2021. As shown in our study, the overlap between coronary ostium and THV commissure, resulting from commissural misalignment, was the most important contributor to THV-related challenging coronary access. The newly introduced promising method, aligning the THV commissures to native commissures during TAVR, may play a critical role in the simplification222324. However, its usefulness may be challenged in BAV patients, especially in type 0 BAV, in which the configuration of commissures and/or the coronary take-off angle significantly differ from those of typical TAV. Hence, this technique needs further evaluation for its feasibility in BAV patients. For patients with bulky native leaflets and low coronary ostium height, not only should the risk of coronary obstruction be considered, but the risk of challenging coronary access should also be factored into TAVR decisions. Additionally, THV designed with a low skirt, small commissural post, and large open cells can directly decrease the potential interaction. THV type selection is essential for simplification as well.
To the best of our knowledge, no study has investigated the optimal fluoroscopic viewing angles for coronary access after TAVR. The optimal projection curve, however, was widely used to provide the patient-specific coplanar view of the aortic annulus during TAVR procedures and was associated with improved outcomes1525. It was also recently suggested for guiding coronary intervention in general patients1726. CT simulation analysis can provide the features of the path to the coronary ostia after TAVR but using this information to guide coronary engagement requires clear identification of relevant anatomical or THV-related structures in fluoroscopic views. The optimal viewing angles may enhance feasibility, and we reported the individual optimal viewing angles for coronary access after TAVR for the first time. We found that the theoretical optimal fluoroscopic viewing angles for coronary access after TAVR were highly variable among the type 0 BAV, type 1 BAV, and TAV groups, not all CT-identified optimal viewing angles were practical (especially for RCA access) using current C-arm equipment, and the proportion that can be used to guide RCA access was significantly higher in the type 0 BAV group. This method may further simplify coronary intervention after TAVR, but future studies are required to assess its feasibility.
Study limitations
Our study has several limitations. First, only patients undergoing TAVR with the self-expanding VenusA-Valve were included, and the results should be extrapolated with caution. However, the results may be applicable to patients receiving CoreValve/Evolut THVs whose design features are very similar to VenusA-Valve. Second, this was a retrospective single-centre study and some patients were excluded according to the predefined criteria, which may lead to selection bias. Third, the small number of coronary angiographies or intervention cases after TAVR limited the feasibility of performing complex statistical analysis. The association between the CT-identified coronary access classification and unsuccessful coronary engagement needs to be confirmed in future studies. Finally, the ability of optimal fluoroscopic viewing angles to guide coronary access was not validated, and future studies that compare its performance with the empirical method should be conducted.
Conclusions
Coronary access may be challenging or complex in a significant proportion of both BAV and TAV patients after TAVR using a self-expanding THV. Type 0 BAV anatomy may be more favourable for coronary access after TAVR. Commissural alignment techniques and THVs designed with low skirts, small commissural posts, and larger open cells may be desirable to simplify future coronary access for BAV and TAV patients during the first TAVR.
Impact on daily practice
Coronary access may be challenging or complex in a significant proportion of both bicuspid and tricuspid aortic stenosis patients after TAVR, although type 0 bicuspid aortic valve anatomy may be more favourable for post-TAVR coronary access. Commissural alignment techniques and transcatheter heart valves designed with low skirts, small commissural posts, and larger open cells may be desirable to simplify future coronary access for BAV and TAV patients during the first TAVR.
Funding
This work was supported by the National Natural Science Foundation of China (81970325, 82170375, 81901825, and 82102129); West China Hospital “1·3·5” Discipline of Excellence Project: “Percutaneous transcatheter aortic valve implantation” and “Mechanisms of aortic stenosis and the clinical applications”.
Conflict of interest statement
M Chen and Y. Feng are consultants for Venus MedTech, the manufacturer of VenusA-Valve. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

