Abstract
Transcatheter aortic valve implantation (TAVI) has revolutionised the treatment of severe, symptomatic aortic stenosis and it is now a proven and effective alternative to surgery for patients regardless of preoperative risk stratification. Nevertheless, the consequent expansion towards younger patients with longer life expectancy focuses attention on long-term considerations. In particular, although the prevalence of coronary artery disease has been shown to decrease with the lowering of estimated risk stratification, the chance of requirement of future coronary interventions after TAVI increases dramatically as a function of patients’ life expectancy. To date, however, only a few studies have investigated the feasibility and reproducibility of coronary artery cannulation after TAVI. Different conditions related mainly to aortic root anatomy and specific transcatheter aortic valve (TAV) designs and deployment have been associated with impaired coronary access after TAVI. In the present review, we will examine the conditions that may make coronary access after TAVI more challenging or even impossible.
Introduction
Transcatheter aortic valve implantation (TAVI) is going to expand its indications towards younger patients1,2,3. These patients have a longer life expectancy compared to those treated previously, which focuses attention on different long-term considerations4. In this setting, transcatheter aortic valve (TAV) durability has already been shown to be comparable to that of surgical valves up to ten years5 and repeat TAVI shown to be a feasible option in case of TAV degeneration. So far, an underestimated aspect has been the necessity to guarantee an easy coronary re-access after TAVI. Furthermore, the probability of the necessity of future coronary interventions is dramatically higher for younger patients, as it rises as a function of life expectancy. This population, particularly when it is affected by concomitant coronary artery disease (CAD), might get more benefit from an easy coronary re-access after TAVI.
Therefore, currently there is a growing interest in investigating the feasibility of coronary cannulation after TAVI, and how to reduce the risk of unsuccessful coronary re-engagement6. This review aims to summarise literature data about coronary access after TAVI and examine the underlying conditions that make coronary re-engagement more challenging or even impossible.
CORONARY ARTERY DISEASE IN PATIENTS WITH SEVERE AORTIC STENOSIS
Aortic stenosis (AS) and CAD frequently coexist, as they share similar risk factors and pathogenesis. It has been reported that up to 80% of patients with AS undergoing TAVI have concomitant CAD7. Historically, patients undergoing surgical aortic valve replacement (SAVR) with concomitant CAD were treated with coronary artery bypass grafting (CABG) at the same stage as SAVR8. Since the advent of TAVI for the treatment of severe AS, the optimal management of concomitant CAD has remained a matter of debate. Several observational studies evaluating the impact of CAD in patients undergoing TAVI have reported controversial results. This can be related to the heterogeneity of the definition of CAD, to the revascularisation completeness, and to the physiological assessment of CAD severity using fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR)9,10. In this setting, the ACTIVATION randomised trial recently compared preprocedural percutaneous coronary intervention (PCI) versus no PCI in patients undergoing TAVI, who had significant CAD without angina11. At one year, the primary endpoint of death/rehospitalisation had occurred in 41.5% of the patients who underwent preprocedural PCI and in 44% of patients who did not. Those event rates were similar, although the bar set for non-inferiority was not met. Besides, if on the one hand pre-existing obstructive or severe coronary stenosis is commonly treated either before or during the TAVI procedure, on the other hand mild CAD is usually left untreated and may also worsen and need treatment later after TAVI. Indeed, the prevalence of symptomatic CAD increases monotonically with age, and a recent analysis reported a rate of post-TAVI acute coronary syndrome of about 10% over two years12. As a result, with the exponential increase in TAVI procedure numbers and the widening of TAVI indications to younger patients, the necessity of performing coronary angiography (CA) and PCI after TAVI is expected to increase dramatically in the near future.
FACTORS INFLUENCING CORONARY RE-ACCESS
The feasibility of coronary cannulation after TAVI is influenced by both TAV-related and patient-related characteristics (Figure 1). The main factors that impact on the feasibility of coronary re-access after TAVI are: 1) the spatial relationship between the TAV stent and aortic wall; 2) the position of the bioprosthesis leaflets; 3) the final position of the bioprosthesis commissures towards the coronary ostia; and 4) the specific design of the TAV (Figure 2). In a prospective study involving 300 consecutive patients undergoing TAVI, our group showed that CA after TAVI using diagnostic catheters was unsuccessful in 7.7% of cases; selective coronary engagement failure was observed almost exclusively with the Evolut™ TAV (Medtronic, Minneapolis, MN, USA, 22 of 23 cases). Furthermore, the combination of the use of the Evolut™ R/Evolut™ PRO (both Medtronic), the oversizing of the TAV compared to the sinus of Valsalva (SoV) dimensions, and a higher TAV implant depth, was shown to predict the risk of unsuccessful coronary cannulation after TAVI with high accuracy13. In the case of a narrow SoV, there will be little remaining space behind the stent frame to manoeuvre the catheter when engaging the coronary ostium. Therefore, catheter manoeuvring will be limited, and it will be more challenging to achieve the selective engagement of the ostia. When considering future valve-in-valve procedures, the short horizontal distance from the TAV frame to the aortic wall may increase the risk of coronary obstruction14,15. Besides, the importance of achieving a lower TAV implantation depth to guarantee coronary access should be carefully evaluated during procedural planning. Indeed, a lower positioning increases the risk of permanent pacemaker implantation and significant paravalvular regurgitation, which in turn may affect long-term outcomes16,17. Although it provides indisputable haemodynamic advantages, the supra-annular position of bioprosthetic leaflets is an adverse factor considering coronary re-access. Available data have shown a lower rate of selective CA after TAVI and after TAVI-in-TAVI with supra-annular devices compared to intra-annular ones18. This is probably related to the relative reduction of coronary ostia height from the neo-annulus and therefore the higher position of neo-commissures, which in turn might prevent the catheter from passing through a favourable stent cell that lies in front of the coronary ostia. Finally, the positioning of one of the bioprosthetic commissures in front of the coronary ostia clearly represents a physical obstacle to achieving coronary cannulation after TAVI. Unlike SAVR, the commissural orientation of the TAV is random because there is currently no reliable method to align the commissures with the native commissures. In a post-TAVI computed tomography (CT) analysis, Ochiai et al demonstrated that, if the coronary ostia are below the skirts or in front of the commissural diamonds or tabs, coronary re-access is more challenging15. However, Tang et al19 have recently evaluated the impact of initial deployment orientation of the most used TAVs on their final orientation and neo-commissural overlap with coronary arteries; however, these techniques require further evaluation regarding safety and feasibility. In the following paragraphs, we will examine the specific design characteristics of the various TAVs and the implantation refinements that could be implemented to increase the possibility of easier coronary access after TAVI.
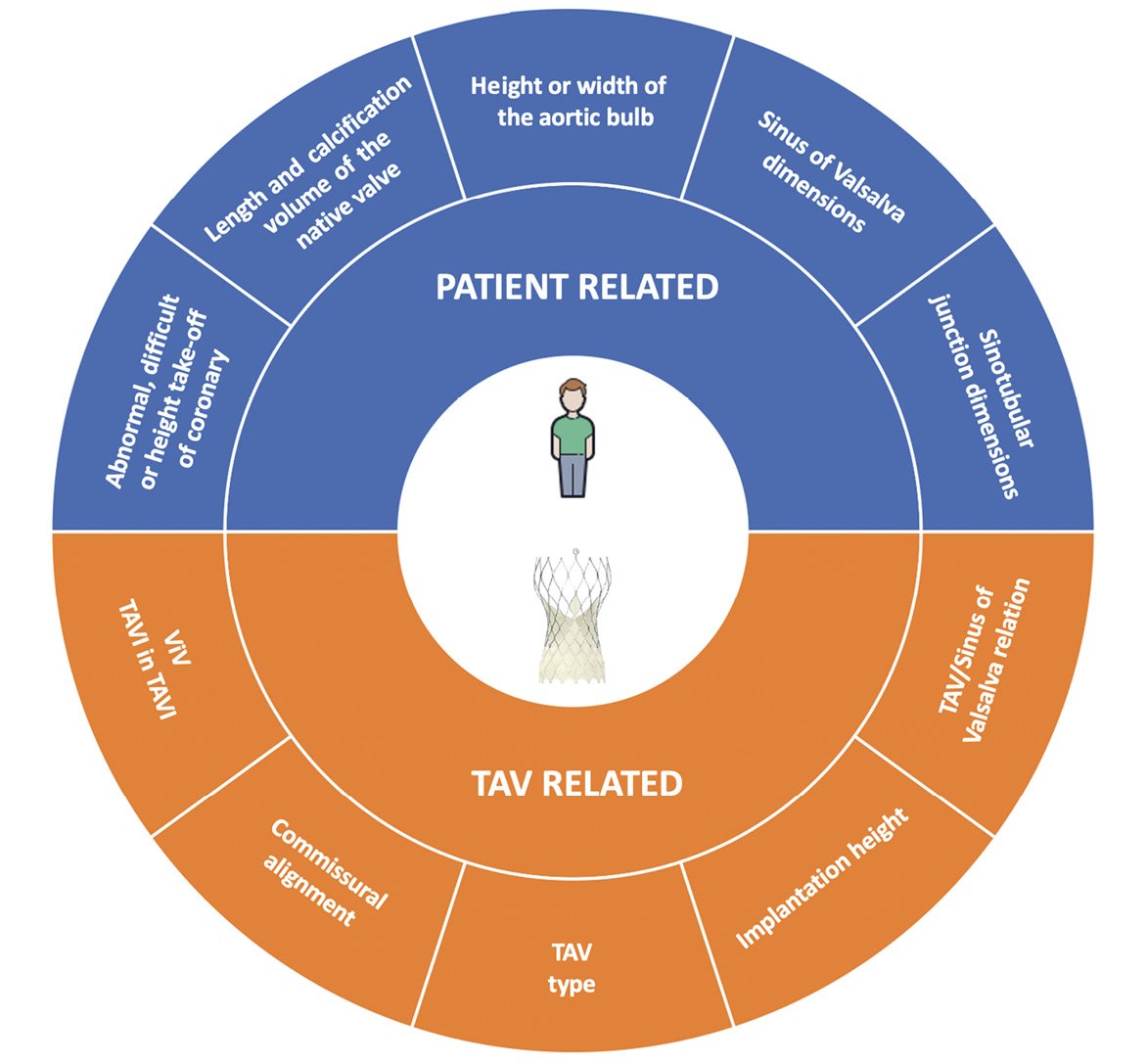
Figure 1. Summary of factors that could impact on coronary re-access after transcatheter aortic valve replacement. TAV: transcatheter aortic valve; ViV: valve-in-valve
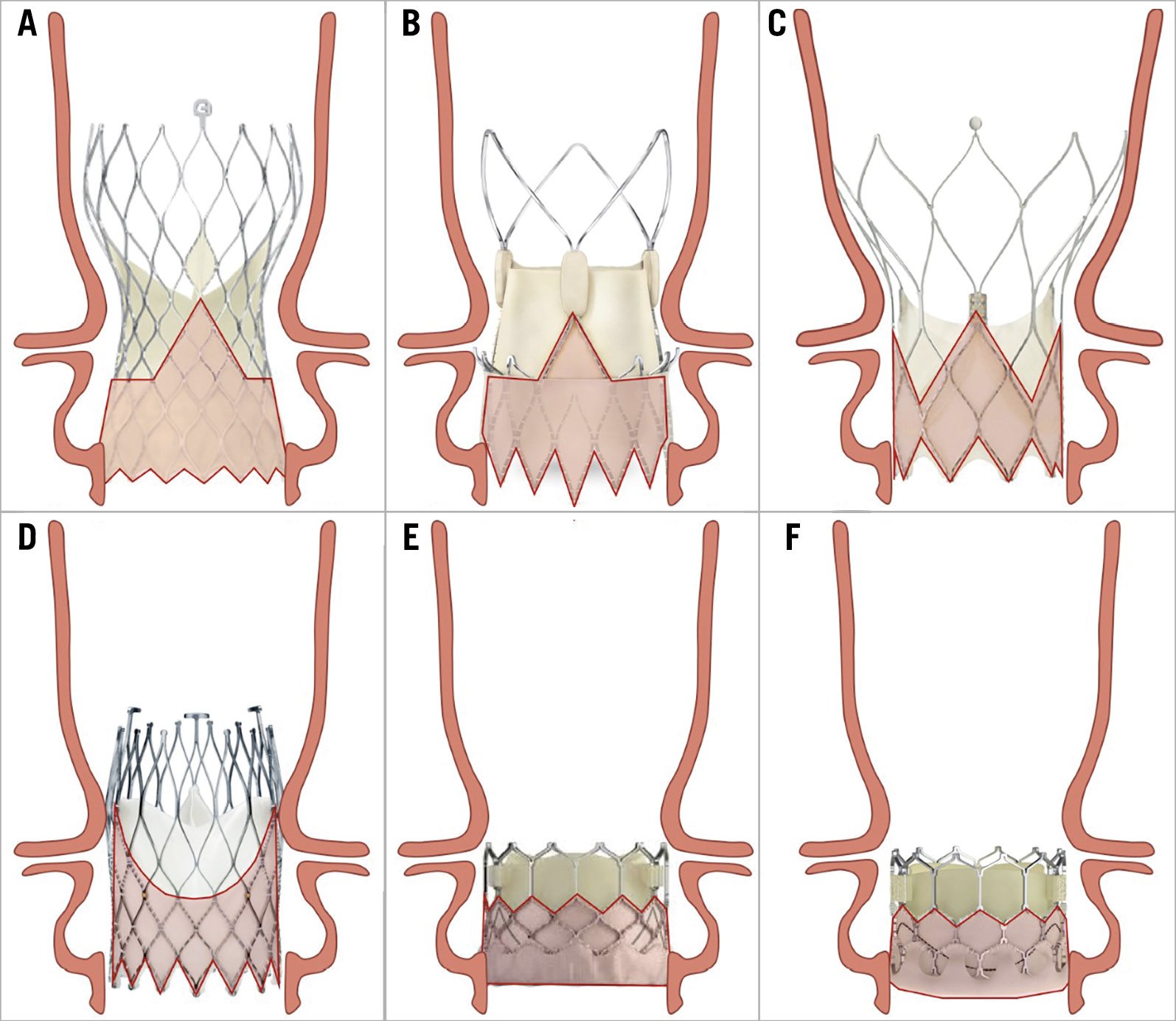
Figure 2. Relationship between aortic root anatomy and transcatheter aortic valve designs. A) Evolut R/PRO/PRO+. B) ACURATE neo/neo2. C) Portico. D) ALLEGRA. E) SAPIEN 3/ULTRA. F) Myval.
SELF-EXPANDING VALVES
EVOLUT R, EVOLUT PRO AND EVOLUT PRO+
The Evolut devices are repositionable, self-expanding (SE) TAVs comprising a trileaflet porcine pericardial tissue valve, sutured in a supra-annular position within a nitinol frame with close, diamond-shaped cells. The Evolut valve is composed of three segments: A) an outflow tract, which measures 32 to 38 mm, that is the largest part of the frame and ends at the level of the ascending aorta; B) a concave central portion, that measures 20 to 24 mm; C) an inflow tract, that measures 23 to 34 mm, which has high radial force and secures the frame within the native annulus. The latter has a sealing skirt that measures 13 to 14 mm (Figure 3). The Evolut PRO/PRO+ are based on the prior Evolut R platform with the addition of an external pericardial wrap, designed to mitigate paravalvular leakage (PVL)20.
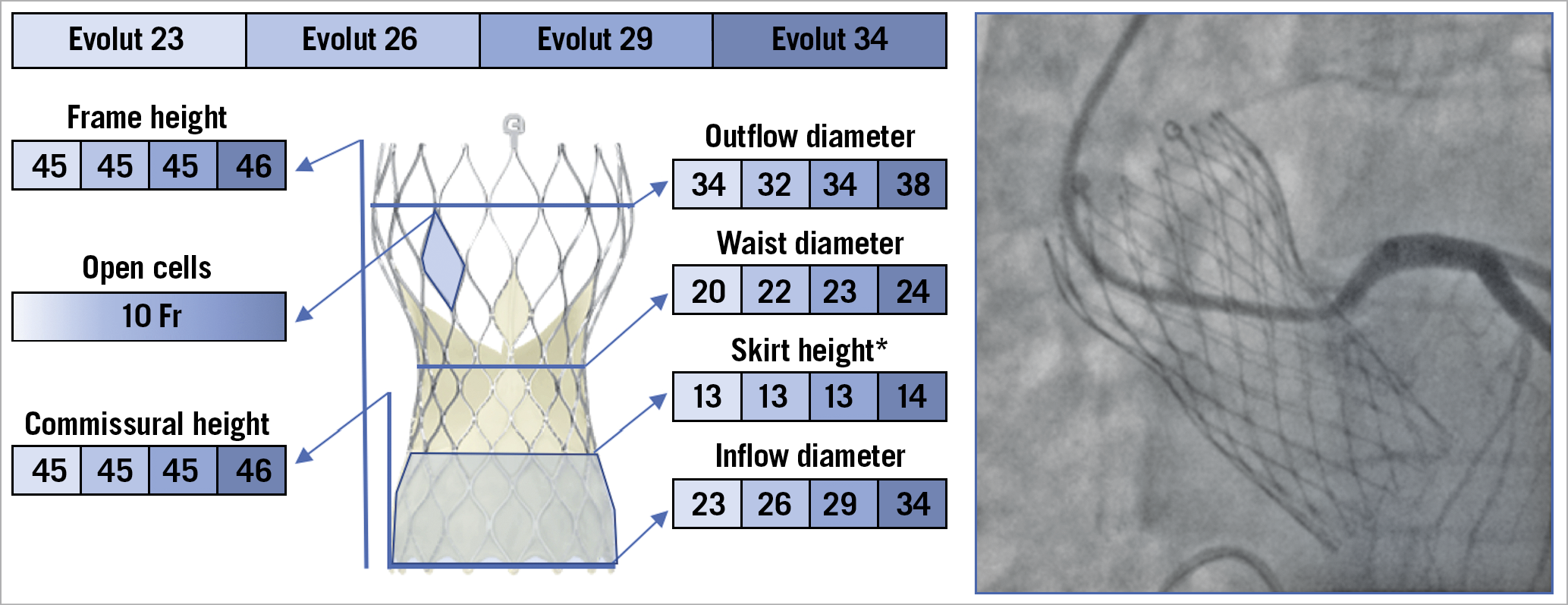
Figure 3. The various features and dimensions of the Evolut TAVs and an example of selective coronary cannulation. All measurements are expressed in millimetres. *For Evolut PRO and Evolut PRO+. Fr: French
A proper implantation depth of this device is desirable, especially in patients with low coronary ostia (<12 mm)21 and SoV diameter <30 mm22, in order to avoid coronary occlusion and to facilitate coronary re-access. The Evolut devices are recapturable until 70-80% of deployment, and it is possible to reposition the device to achieve the intended implantation depth. The Evolut valve frame is covered by a pericardial skirt, that extends from the inflow portion up to the level of the leaflets. Due to the supra-annular position of the leaflets, it is important to avoid a high final position of the valve in case of borderline coronary height. In this setting, it is desirable to position the valve approximately 4 mm to 6 mm below the annulus (midway between node 0 and node 1 of the frame of the bioprosthesis). If the TAVs are deployed too high and/or the coronary arteries are too low, selective coronary cannulation could be particularly challenging as the catheter should enter a diamond above the level of the ostium and engage it pointing downwards.
Given the valve design, misalignment of bioprosthetic commissures plays an important role with regard to the feasibility of coronary re-access after TAVI. The C-tab paddle corresponds to one of the commissures and is aligned with the flush port of the delivery system during valve mounting, introduced facing the anterior part of the ascending aorta. In the standard technique, the delivery system is inserted with the flush port at 12 o’clock. Recently, Tang et al evaluated the incidence of post-TAVI commissural misalignment according to C-tab marker orientation. The patients with the C-tab marker at the outer curve and the centre front of the aortic root during the initial deployment had improved commissural alignment and a lower incidence of severe coronary ostia overlap than those who had a C-tab marker at the inner curve and centre back (p<0.001 for all) with the left main (LM) coronary artery (15.7% vs 66.0%), right coronary artery (RCA) (7.1% vs 51.1%), both coronary arteries (2.5% vs 40.4%), and one or both coronary arteries (20.2% vs 76.6%). They showed that inserting the delivery system with the flush port at the 3 o’clock position, away from the operator, results in the C-tab marker position being at the outer curve of the aortic root during TAV deployment. As a consequence, these patients had improved commissural alignment and a lower incidence of severe coronary artery overlap with LM, RCA, both coronary arteries, and one or both coronary arteries (p<0.001 for all)19 (Figure 4).
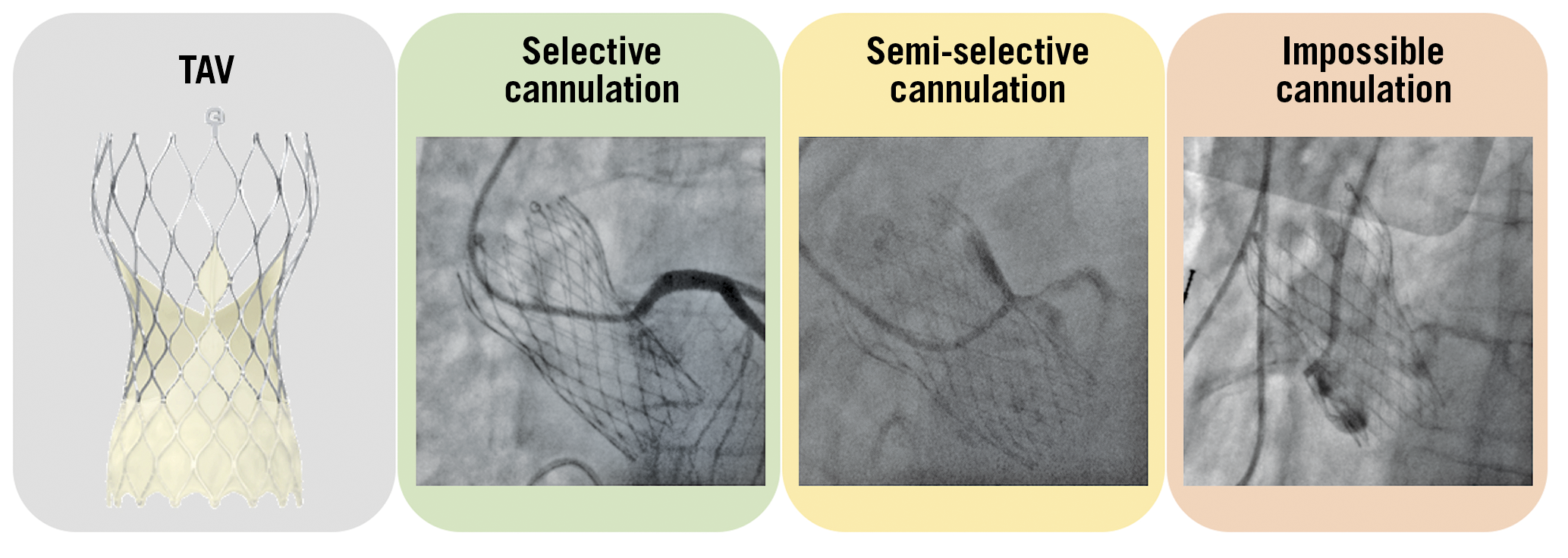
Figure 4. Examples of selective, semi-selective and impossible coronary ostia cannulation with an Evolut R/PRO transcatheter aortic valve. TAV: transcatheter aortic valve
Unlike balloon-expandable (BE) TAV, additional steps are taken when implanting an Evolut TAV. The cusp overlap view by overlapping the right coronary cusp and left coronary cusp isolates the non-coronary cusp on the left-hand side of the screen and offers several advantages. This view provides a good anatomical reference for deployment depth as it elongates the outflow tract in a long-axis view and reduces or eliminates parallax of the delivery catheter. In addition, this view guarantees the optimal projection to confirm a good commissural alignment23.
ACURATE neo AND ACURATE neo2
The ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) is composed of an SE nitinol stent frame with three porcine pericardial leaflets in the supra-annular position. The frame consists of a lower crown at the inflow portion, which fixes the device inside the native annulus and protrudes a few millimetres into the left ventricular outflow tract, an upper crown, that is designed to capture and push down the overturning, native leaflets, and three stabilisation arches at the outflow portion, which enhance co-axiality and stabilisation during deployment. Its open-cell frame is designed to ensure an easier access to coronary arteries after the implantation and reduce the risk of coronary obstruction in patients with low coronary height, small SoV, and/or long, calcific leaflets. It is available in three sizes –S, M, and L– to treat patients with aortic annulus diameters from 21 mm to 27 mm (S from 21 mm to 23 mm, M from 23 mm to 25 mm, and L from 25 mm to 27 mm)24. Although it is not repositionable, this device has a unique two-step, top-down releasing mechanism that allows a stable positioning without rapid ventricular pacing.
Due to the supra-annular position of the leaflets and therefore the high position of the commissures, a severe overlap of neo-commissures with the coronary ostia could be an obstacle to coronary re-access. Tang et al reported a severe overlap of ACURATE neo commissures in 28.0% with the LM, in 42.0% with the RCA, in 19.0% with both coronary arteries, and in 51.0% with one or both coronary arteries. The ACURATE neo orientation can be guided by the three commissural posts on fluoroscopy. They demonstrated that the incidence of coronary ostia overlap was lower when the commissural post was at the inner curve or centre back of the aortic root during deployment. Recently, it has been shown that an accurate commissural alignment can be achieved by orienting two overlapping commissural posts at the outer curve and the remaining commissural post at the inner curve in the cusp overlap view25. Nevertheless, currently there is no validated method to achieve this intended alignment without torqueing manoeuvres of the delivery system.
Recently, the ACURATE neo2 system received a CE mark (Figure 5). This new iteration of the device is based on the previous ACURATE neo platform with the implementation of new features to improve valve haemodynamic performance, such as the extended sealing skirt with the new Active PVseal™ technology (Boston Scientific) which aims to minimise paravalvular regurgitation.
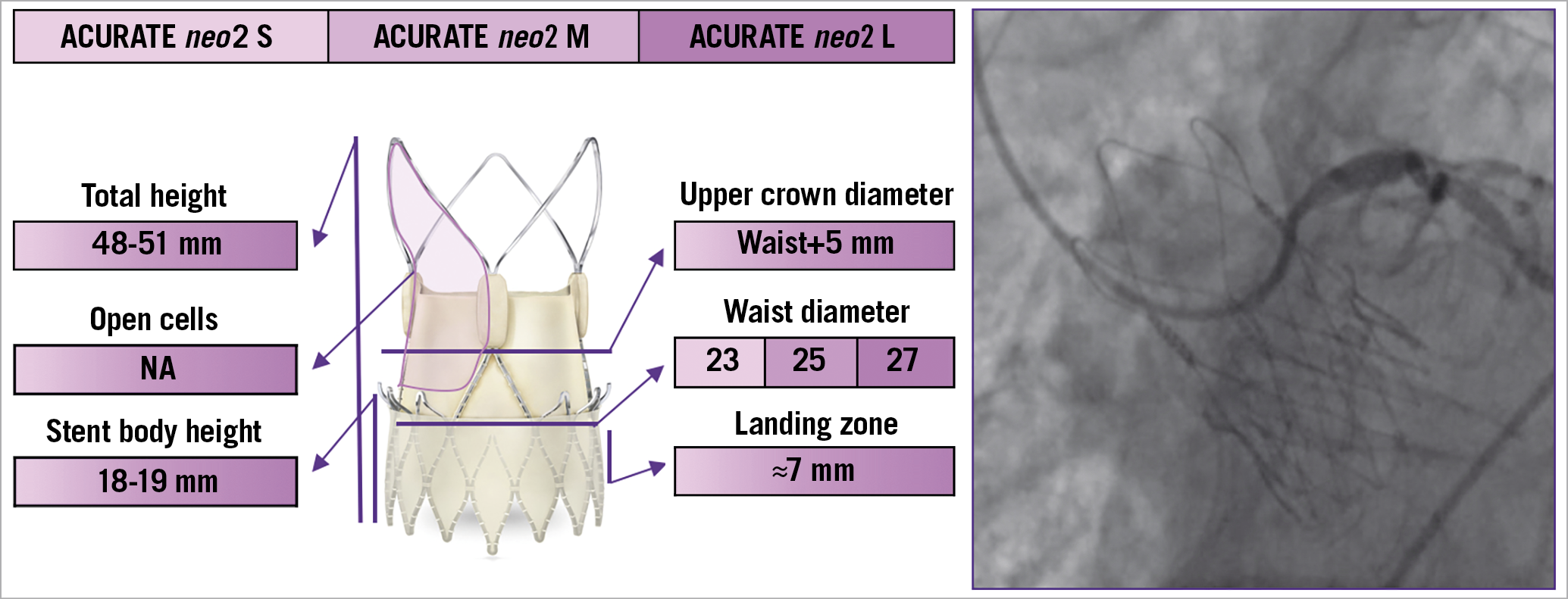
Figure 5. The various features and dimensions of the ACURATE neo2 and an example of selective coronary cannulation. It is available in three sizes - S, M, and L - to treat patients with aortic annulus diameters from 21 mm to 27 mm. NA: not available
ALLEGRA
The ALLEGRA TAV (New Valve Technology, Hechingen, Germany) is a new SE valve that consists of a nitinol stent frame and bovine pericardial leaflets in a supra-annular position. The stent frame has a large, diamond-shaped cell configuration with a 12 mm bovine pericardial sealing skirt at the inflow portion that reduces the risk of significant PVL. Six radiopaque gold markers are incorporated into the stent frame at the level of the annular plane to guide a proper valve positioning, which is particularly useful in the setting of valve-in-valve TAVI. The unique PermaFlow® (New Valve Technology) releasing mechanism ensures stability of the device during deployment and a minimal impact on patient haemodynamics, as the valve is already functioning during the early phase of release. The ALLEGRA valve is available in three sizes (23, 27, and 31 mm), with a frame height of 37.3, 41.3, and 43.0 mm, respectively, covering aortic annulus diameter sizes from 19 to 29 mm26 (Figure 6). The design of this device is thought to facilitate coronary re-access after TAVI, and the possibility of identifying the exact position of the neo-annulus with the six radiopaque gold markers is particularly helpful in case of low coronary height and small SoV. However, no data about coronary re-access with this valve are currently available.
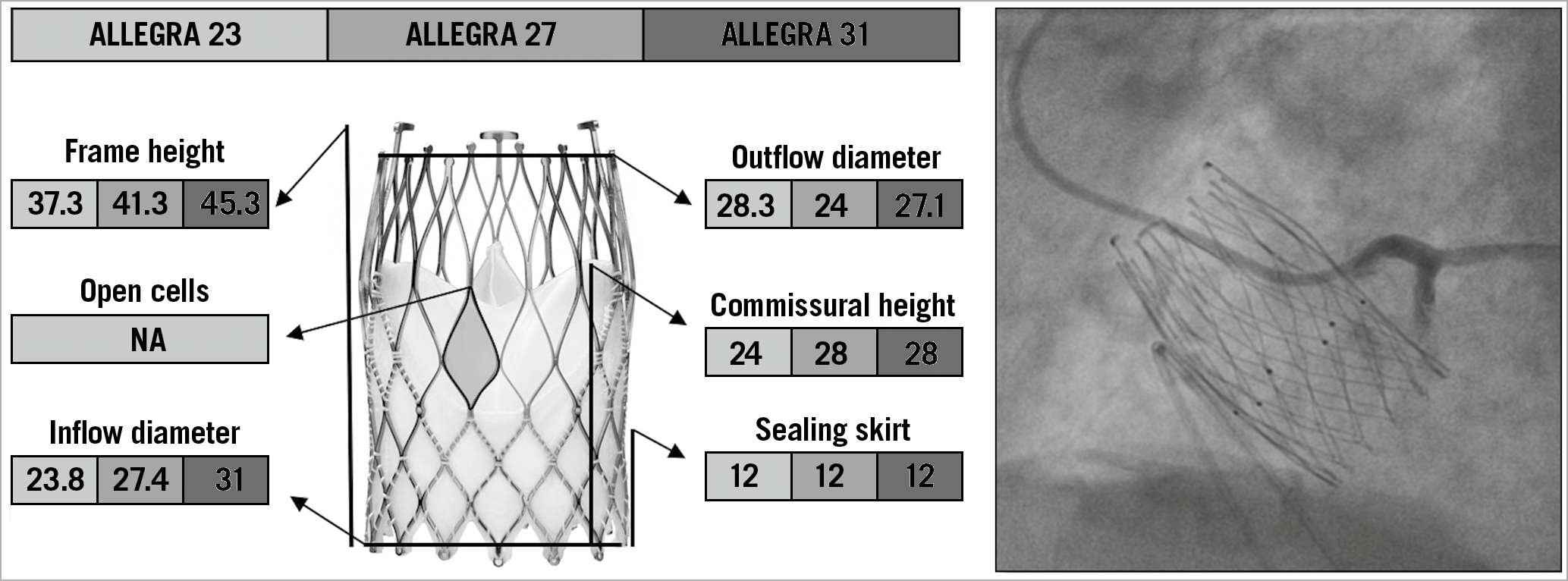
Figure 6. The various features and dimensions of the ALLEGRA and an example of selective coronary cannulation. All measurements are expressed in millimetres. NA: not available
PORTICO
The Portico™ TAV (St. Jude Medical, St. Paul, MN, USA) is a nitinol-based SE valve with a porcine pericardial sealing cuff and intra-annular bovine pericardial leaflets27 (Figure 7). The outflow portion of the stent frame incorporates three retention tabs that indicate the three commissures. There are four sizes of the Portico valve, measuring 23 mm, 25 mm, 27 mm, and 29 mm at the inflow level28. The ideal depth of implantation is represented by the frame’s inflow edge placed 3-4 mm below the aortic annulus. If a suboptimal implantation height is achieved initially, the valve can be partially re-sheathed and repositioned. Although it has a structure similar to that of the Evolut valve, the Portico TAV has larger cells, and the intra-annular position of the bioprosthetic leaflets represents a clear advantage for coronary re-access after TAVI. Indeed, the lower position of commissures does not impair coronary re-engagement when the native coronary height is relatively low, unless a commissural post faces a coronary ostium directly. Tagliari et al first showed that commissural alignment with the Portico valve is feasible and reproducible in specific 3D printed aortic models of patients29. Similar to the ACURATE neo TAV, the use of the cusp overlap view allows proper alignment of the neocommissures with the native ones, by isolating them at the inner aortic curve25.
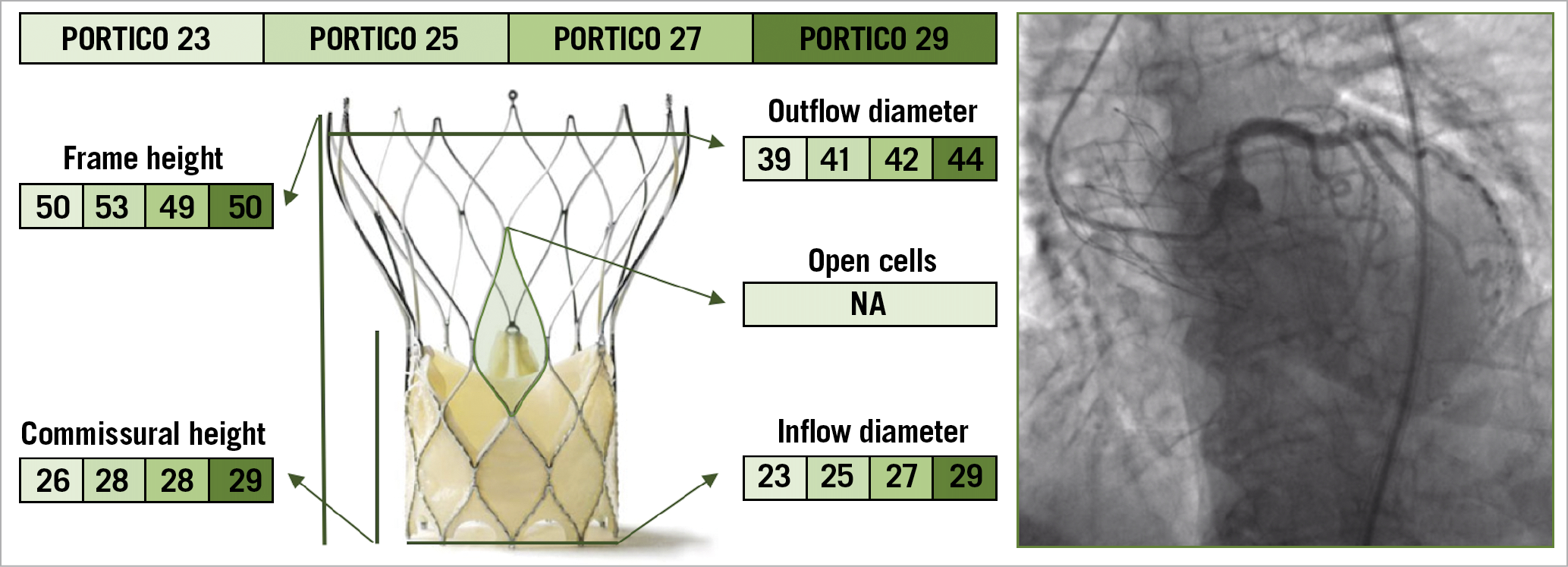
Figure 7. The various features and dimensions of the Portico and an example of selective coronary cannulation. All measurements are expressed in millimetres. NA: not available
BALLOON-EXPANDABLE VALVES
SAPIEN 3 AND SAPIEN 3 ULTRA
The SAPIEN 3 TAV (Edwards Lifesciences, Irvine, CA, USA) is composed of a cobalt-chromium frame with a polyethylene terephthalate fabric skirt at the inflow portion, into which are mounted three leaflets of bovine pericardial tissue. To enhance the sealing mechanism further and mitigate PVL, its iteration, the SAPIEN Ultra, features a textured outer portion of the polyethylene terephthalate material that has a greater height compared to the previous TAV. The frame has 12 large cells in the upper row, three of which contain the commissural attachment (marked by three radiopaque double lines), corresponding to a 3 mm pledget at the outer part of the stent. Compared to the previous generation, the upper cells of SAPIEN 3/Ultra TAVs are 38% larger in area, ensuring an easier engagement of the coronary ostia30 (Figure 8, Figure 9).
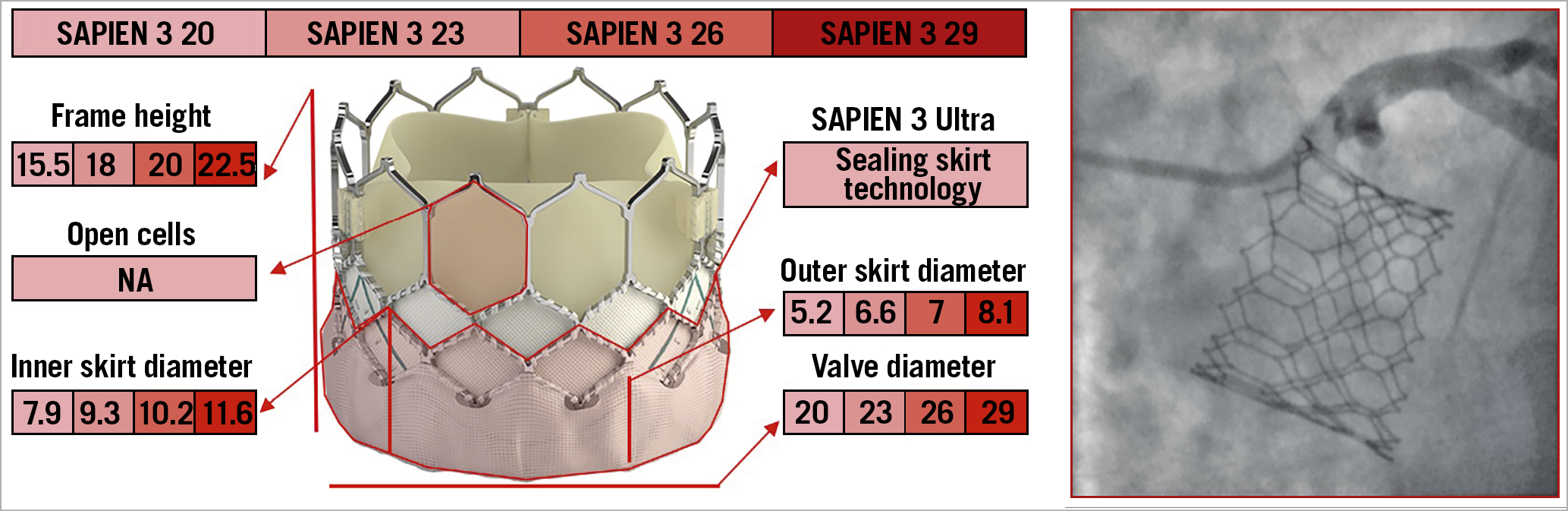
Figure 8. The various features and dimensions of the SAPIEN 3/Ultra and an example of selective coronary cannulation. All measurements are expressed in millimetres. NA: not available
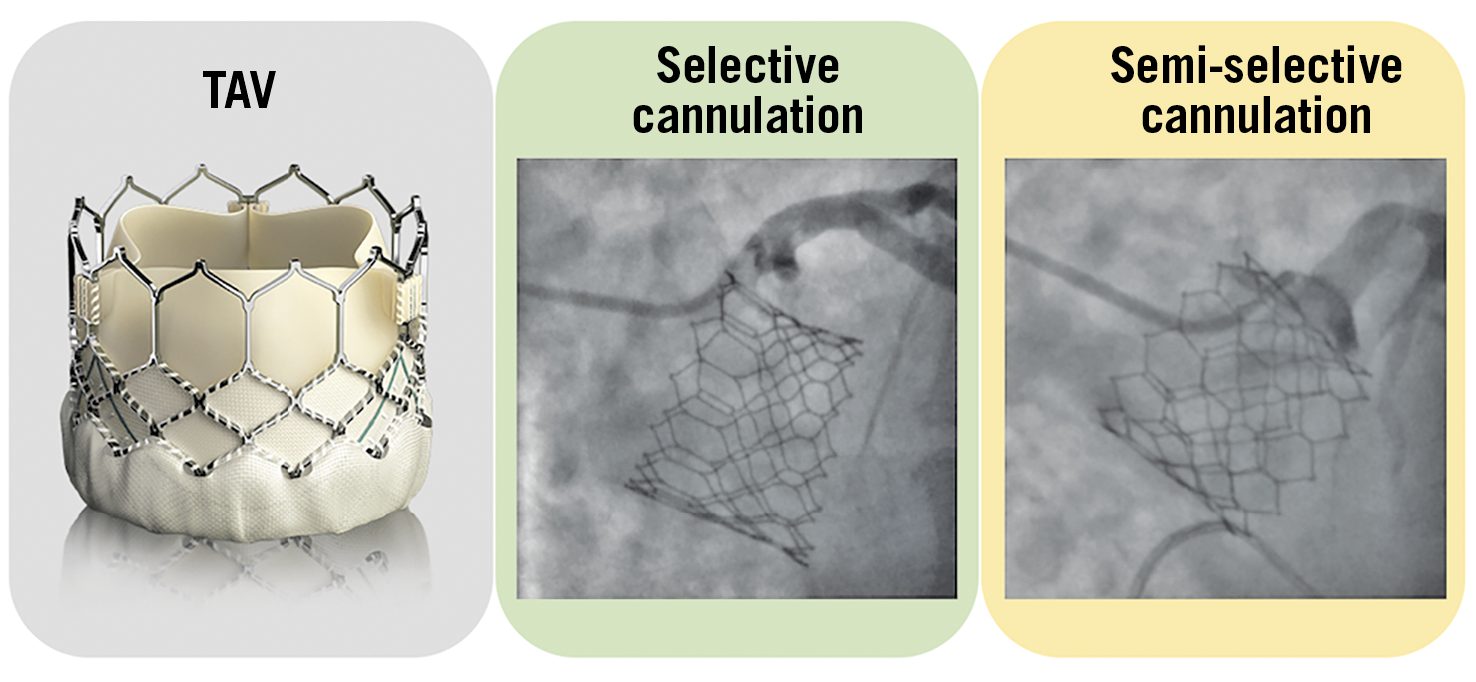
Figure 9. Examples of selective and semi-selective coronary ostia cannulation with a SAPIEN 3 transcatheter aortic valve. TAV: transcatheter aortic valve
Balloon-mediated valve deployment allows accurate and controlled positioning of the device. The height of the implantation can be carefully controlled, with the central radiopaque marker corresponding to the centre of the delivery balloon on which is centred the crimped valve. Furthermore, the fine-tuning knob on the delivery system allows millimetric adjustment of valve positioning just before implantation. Currently, it is recommended to place the bottom of the central marker at the ideal line crossing the base of the three native cusps or slightly above, thus aiming at a higher implant (80/20 or 70/30 aortic/ventricular ratio) to reduce the incidence of conduction disturbances and paravalvular regurgitation31. Although the SAPIEN 3/Ultra valve does not have a narrowed waist like the Evolut TAV, the shorter stent frame, and the large upper row cells allow an easy coronary re-access, that can be mostly achieved from above the TAV stent.
Nevertheless, the presence of bioprosthetic commissures in front of the coronary ostia can represent an obstacle to coronary re-access, especially when the SoV is small and the stent frame faces the aortic wall above the sinotubular junction (STJ). Unfortunately, a reliable method to orientate commissural alignment with the SAPIEN 3/Ultra valve has not been demonstrated19.
MYVAL
The Myval (Meril Life Sciences, Vapi, Gujarat, India) is a BE TAV composed of a nickel-cobalt stent frame with a complete honeycomb design and a double inner and outer sealing skirt, and a bovine pericardial leaflet sealed in an intra-annular position. The open, large cells on the upper half ensure an easier coronary access, in a manner very similar to that of the SAPIEN 3/Ultra valve. Nine different device sizes are available, with the smallest valve measuring 20 mm and the largest measuring 31 mm (Figure 10). Differently from other TAVs, the Myval is available in intermediate sizes with 1.5 mm increments, which allows a proper size selection for each anatomy, avoiding extreme undersizing or oversizing of a given device. To date, the experience with this TAV is very limited32. Due to the similarity in design and implantation technique with the SAPIEN 3/Ultra valve, coronary access after TAVI is not expected to be particularly challenging with this valve.
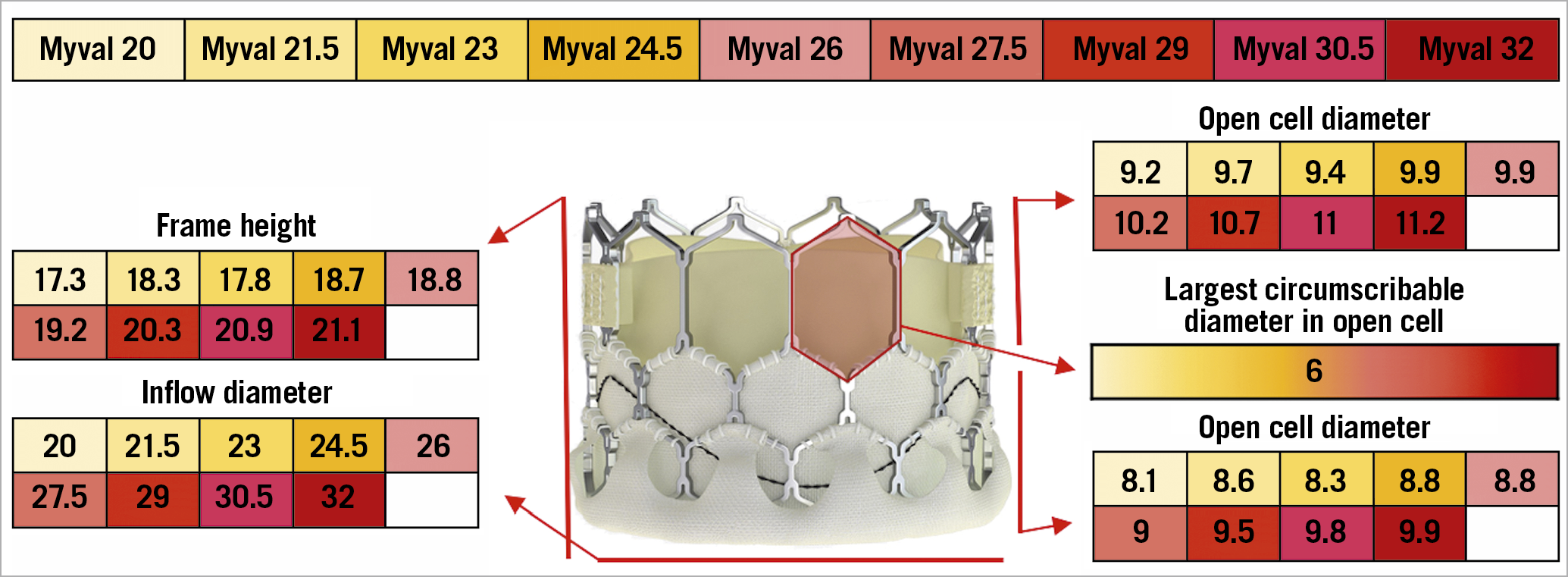
Figure 10. The various features and dimensions of Myval. All measures are expressed in millimetres.
CORONARY RE-ACCESS STRATEGY AFTER TAVI
It is essential to understand the potential challenges and technical implications of CA and PCI in patients who underwent TAVI, for a quick and selective coronary ostia cannulation, especially in the setting of acute coronary syndromes (Central illustration). Recently, the TAVRCathAID application was released on both Google Play Store and Apple Store. It is an educational mobile app with illustrations of the relationship between the coronary ostia and TAV in different anatomies, and a step-by-step guided approach to performing CA and PCI after TAVI (Bhatheja et al. TAVRCathAID: an educational mobile application to learn coronary access after transcatherter aortic valve replacement. Presented at ACC. 2019, New Orleans, USA, 16-18 March 2019) (Figure 11).
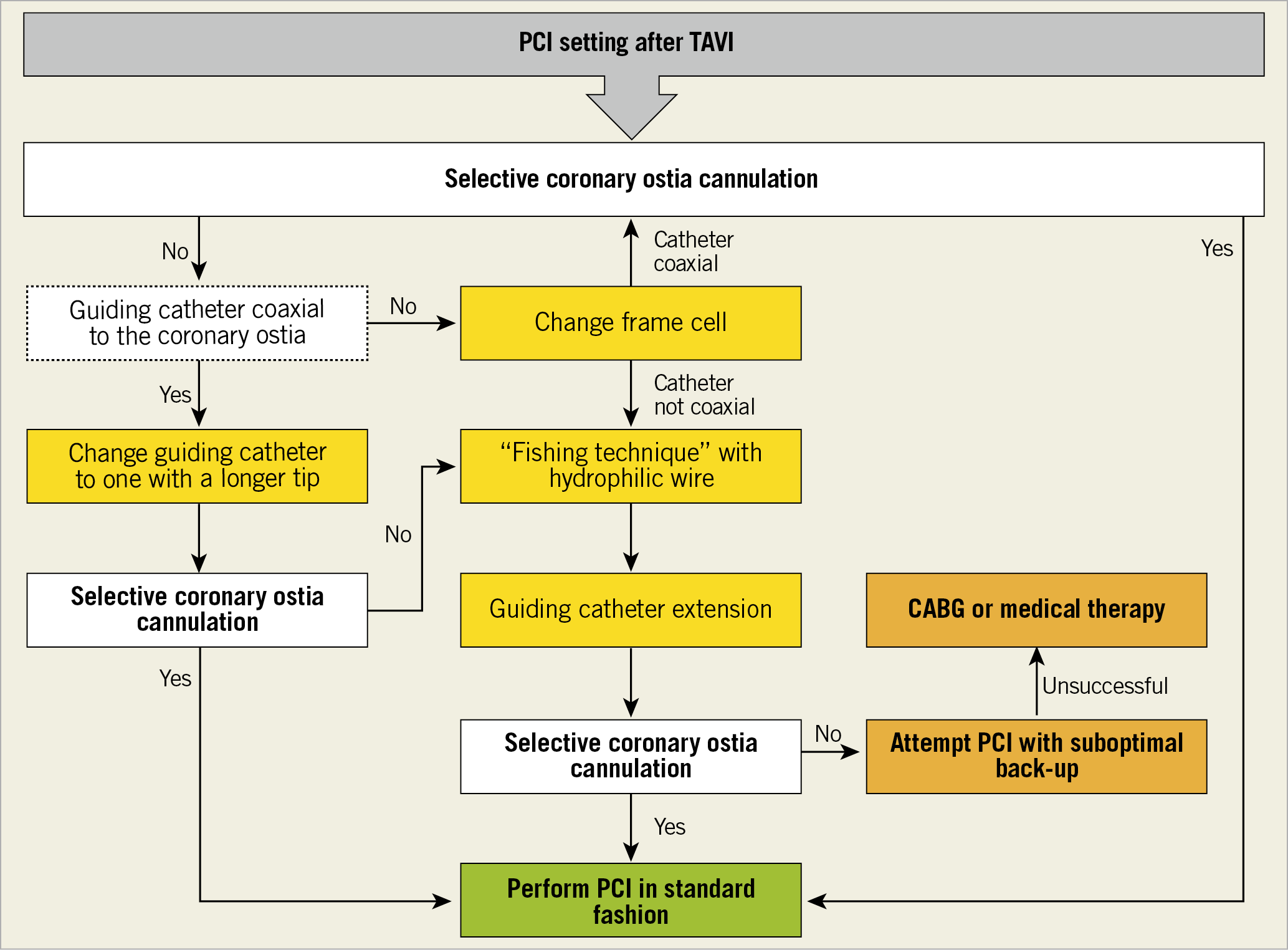
Central illustration. Algorithm for coronary ostia cannulation after TAVI in case of PCI.
Figure 11. TAVRCathAID mobile app.
SELF-EXPANDING TAVs
The coronary ostia cannulation strategy should be modified in the presence of an SE TAV to perform CA or PCI. Because of the difficulties related to the presence of a TAV stent, a femoral or left radial artery access is recommended for CA or PCI, particularly during an acute coronary syndrome. Selective catheter engagement may be difficult due to the position of commissural posts relative to the coronary ostia and the supra-annular position of valve leaflets (Figure 4). Cannulation should be performed in a coaxial manner through the diamond in front of the coronary ostia, especially during a PCI. Indeed, coronary engaging from a diamond below the ostia has been associated with kinking of the guide and the impossibility to remove it33 (Figure 12). Engagement of the LM usually requires a smaller catheter due to the narrow waist of the Evolut TAVs. Downsizing the catheter size by 0.5 cm and maintaining a 0.035'' J-tip wire inside the catheter may assist the coronary cannulation13. If data about catheter choice prior to TAVI are not available, Judkins left (JL) 3.5 and JL3.0 catheters can be used as first-choice catheters for femoral and left radial access, respectively. If the catheter is unable to enter in front of the ostia for geometric interaction with the commissures, the coronary artery should be engaged from another cell adjacent to the commissural post. If it is initially impossible to engage the coronary ostia, a semi-selective angiogram should be performed to guide further attempts. Therefore, guiding catheters (e.g., extra back-up [EBU] 3.0/3.5) with a coronary guidewire and guide extension catheter support could be helpful to obtain selective cannulation. Finally, the anchoring balloon could represent an additional technique to obtain more selective or supportive cannulation if required34,35. Regarding RCA engagement, a standard strategy with a Judkins right (JR) 4.0 should be adopted. Oversizing the catheter size by 0.5 cm or using an Amplatz right (AR) 2 may be helpful with a wide SoV. If a commissural post is in front of the coronary ostium, a multipurpose (MP) catheter or an Ikari right guide should be preferred30.
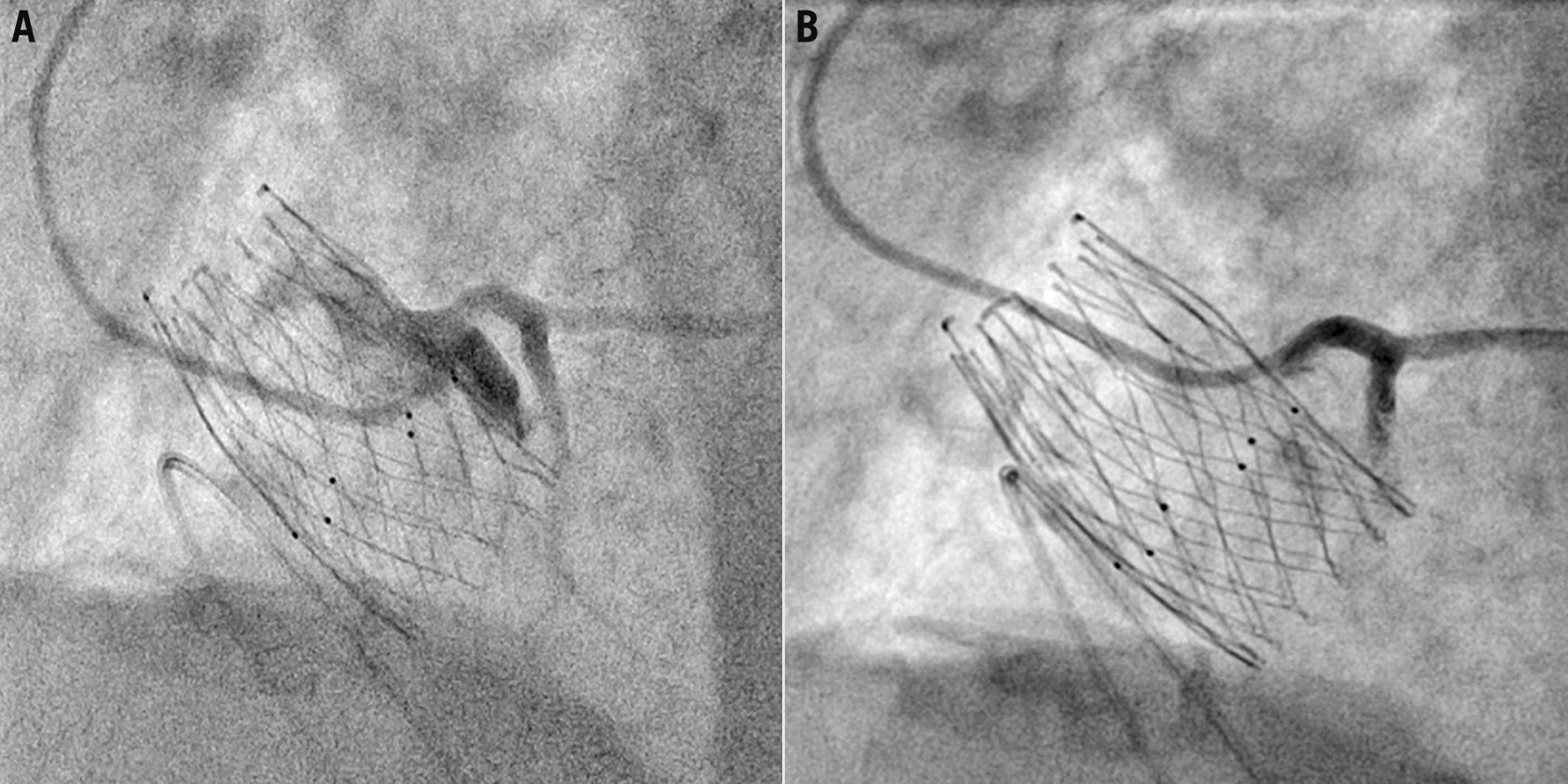
Figure 12. Examples of semi-selective coronary cannulation engaging from a diamond below the coronary ostia (A) and selective coronary cannulation engaging from a diamond in front of the coronary ostia (B) with the ALLEGRA transcatheter aortic valve in the same patient.
BALLOON-EXPANDABLE TAVs
In contrast to SE TAVs, access selection, catheter choice, and coronary ostia engagement technique do not have to be modified in the presence of BE TAVs. CA and PCI may be undertaken in two different ways. In cases where the SoV height is higher than the TAV stent, coronary artery engagement can be achieved from above the SAPIEN 3/Ultra valve. Coaxial engagement would depend on the width of the SoV. If the sinuses are effaced, there will be a relative lack of room to manipulate the catheter and engagement of the artery could be more challenging (Figure 9). In cases where the top of the SAPIEN 3/Ultra stent faces the aortic wall above the STJ, coronary engagement must be through the upper row of cells of the stent. In this setting, if one of the commissural tabs faces the coronary ostium, it is necessary to obtain coronary engagement from an open cell lateral to the commissural tab. If only a non-selective angiogram is achieved, it is necessary to use a coronary wire to engage the artery with a railing technique, as described above. In rare cases, a downward-pointing catheter, such as an MP1, may facilitate engagement from either a cell lying above and not properly aligned with the coronary ostia or from the space between the valve frame and the STJ30,35.
DATA ABOUT CORONARY RE-ACCESS AFTER TAVI
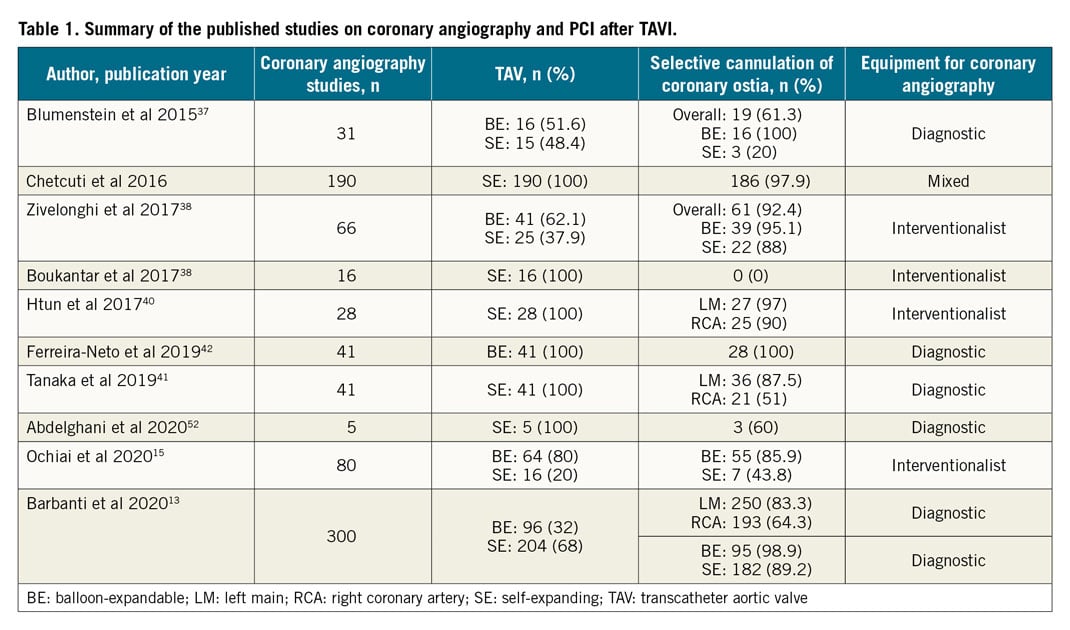
Since the first case report highlighting successful CA and PCI post TAVI with a BE valve in 200734, different studies have reported heterogeneous data about the feasibility of CA or PCI after TAVI (Table 1). Blumenstein et al36 presented results about 35 patients who required CA or PCI after TAVI. Selective cannulation was possible in all patients with a TAV implanted in a sub-coronary position (SAPIEN n=19, JenaValve [JenaValve Technology, Munich, Germany] n=1); however, in patients with TAV types that are placed over the coronary ostia (CoreValve® [Medtronic] n=10, ACURATE n=4, Portico n=1), full accessibility of the coronary ostia was possible in only three. Chetcuti et al reported data of 169 patients who required CA or PCI after TAVI from the CoreValve US trial programme (Chetcuti et al. Percutaneous Coronary Intervention after Self-Expanding Transcatheter Aortic Valve Replacement. Presented at TCT 2016, Washington, DC, USA). Coronary re-access was achieved in 97.9% of cases. Zivelonghi et al38 analysed 66 consecutive patients undergoing TAVI, 41 with BE, and 25 with SE TAVs. RCA and LM cannulation (132 vessels) was successful in all cases except in one left coronary artery after high implantation of an SE TAV (failure rate, 1 in 50 vessels). In 6 vessels (4%), CA was initially non-selective but, after positioning the intracoronary guidewire, selective injections were obtained in all these cases. On the other hand, Boukantar et al39 described data on 16 patients after TAVI with an SE TAV CoreValve. In none of these patients was post-TAVI CA classified as successful. Furthermore, Htun et al40 and Tanaka et al41 showed that the rate of selective right CA was relatively low when compared to the left system (selective CA: LM 42/43, 97% vs RCA 29/32, 90%; LM 36/41, 87.5% vs RCA 21/41, 51%, respectively). With regard to coronary cannulation attempts after TAVI in SAPIEN BE valves, an analysis conducted by Ferreira-Neto et al42 showed that CA and PCI did not exhibit significant changes in technical and equipment procedural aspects such as arterial access site, catheter diameter, number and kinds of catheter needed, procedural duration, fluoroscopy time, contrast volume, and achievement of selective coronary injection compared to the pre-TAVI setting. In line with the previous studies, Ochiai et al15 reported a higher rate of successful selective cannulation after TAVI with intra-annular BE TAVs (BE 55/64, 85.9% vs SE 7/16, 43.8%). Finally, the only available data regarding a systematic attempt to investigate the issue of coronary access after TAVI from real-world practice came from the RE-ACCESS study13. It was the first prospective study investigating the feasibility of a coronary re-engagement after TAVI in real-world practice, using diagnostic catheters. Moreover, it investigated patients undergoing TAVI with all commercially available devices, and it focused on patients’ preprocedural anatomic characteristics and procedural variables. Among 300 patients enrolled, a total of 23 cases (7.7%) of unsuccessful coronary cannulation after TAVI were documented: they occurred in 22 of 23 cases with the use of Evolut R/PRO TAVs (17.9% vs 0.4%; p<0.01). Furthermore, the use an Evolut TAV was independently associated with the risk of unsuccessful coronary cannulation after TAVI, highlighting the importance of valve design in ensuring access to the coronary arteries for either diagnostic or interventionalist future coronary procedures.
CORONARY RE-ACCESS AFTER TAVI-IN-TAVI
With the increasing number of younger patients undergoing TAVI, repeat interventions after TAVI are expected to rise dramatically in the future. TAVI-in-TAVI is a feasible treatment in case of TAV degeneration, although data are relatively limited so far43,44. In these patients, coronary re-access is more challenging than in single TAVI patients because, in addition to the displaced leaflets of the native aortic valve, there will be two metallic frames and a higher probability of having commissural suture posts in front of the coronary ostia. The position of the first TAV is crucial for maximising the possibility of having a selective engagement of the coronary ostia after TAVI-in-TAVI45. The worst scenario is with the TAV in proximity to, in direct contact with, or above the STJ because there is a considerable risk of coronary occlusion. The deployment of a second TAV could cause sequestration of the coronary sinus by the displaced leaflets of the first TAV, making coronary re-access impossible46. In a post-TAVI CT analysis, Buzzatti et al tried to address the issue of the potential unfeasibility of coronary re-access after TAVI-in-TAVI. They defined an increased risk of impaired coronary access if the coronary ostium was below the TAVI commissures with a valve-to-aorta distance <2 mm. They reported an increased risk in more than half of the patients (123/221, 55.6%). A small STJ (STJ width, odds ratio [OR] 0.68, confidence interval [CI]: 0.56-0.81, p<0.001; STJ height, OR 0.81, CI: 0.69-0.95, p<0.011) and supra-annular devices (OR 19.8, CI: 6.6-58.8, p<0.001) were associated with a predicted increased risk47. Recently, De Backer et al analysed CT scans in 45 patients who underwent TAVI-in-TAVI treated with different combinations of CoreValve, Evolut, and SAPIEN TAVs. Coronary ostium below the top of the neo-skirt, a TAV-to-aortic wall distance of <3 mm if the coronary ostium was below the top of the neo-skirt and a distance between the stent struts at the “crossing zone” <3 mm were associated with unfavourable coronary access after TAVI-in-TAVI. As a consequence, the use of a SAPIEN valve as first TAV, which has an intra-annular leaflet position and therefore a lower commissural height, as well as large open stent frame cells, is associated with lower expected device-related interference with coronary access after TAVI-in-TAVI48. Finally, a CT simulation in 81 patients who underwent TAVI with the Evolut PRO confirmed that the use of a second Evolut PRO for TAVI-in-TAVI would hinder future coronary access in up to 78% of patients49. Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVI (BASILICA) is a novel trans-catheter technique performed immediately before TAVI to prevent coronary artery obstruction50. Following the first in vitro data reported by Khan et al, TAVI-in-TAVI BASILICA did not appear effective in reducing coronary obstruction risk and consequently in facilitating coronary cannulation after TAVI-in-TAVI. Effective leaflet splay cannot be achieved in new-generation devices (SAPIEN 3 and Evolut R/PRO) in most cases. Thus, although it may be feasible to create an adequate leaflet splay with BASILICA in some cases, success or failure will depend on commissural alignment and depth of implantation of the new TAVR device51. However, in vivo data are expected to define its role in this scenario. More data are needed to improve the knowledge on the optimal strategy to ensure the feasibility of coronary access after TAVI-in-TAVI.
Conclusion
Currently, estimating the real incidence, feasibility, and success rates of CA and PCI after TAVI is challenging due to the limited available studies regarding this issue. Nevertheless, it seems evident that valve design matters in terms of re-access, because supra-annular, closed stent frame cell, SE TAVs are associated with greater challenges in coronary angiography and PCI post TAVI. Attention should be paid to the choice of a specific TAV for each anatomy and to the proper implantation depth, as well as to the possibility of orientating the bioprosthetic commissures in order to avoid severe overlap with the coronary ostia. In any event, the matter of access to coronary arteries requires further, larger and systematic studies. The development of next-generation TAVI devices that will guarantee an easy, reliable and reproducible access to the coronary arteries is particularly awaited.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences and serves as an advisory board member for Medtronic. He has received speaker honoraria from Medtronic, Abbott Vascular, Edwards Lifesciences and Boston Scientific. C. Tamburino is a consultant for Medtronic. The other authors have no conflicts of interests to declare.
Supplementary data
To read the full content of this article, please download the PDF.

