Coronary access after transcatheter aortic valve implantation (TAVI) procedures can be challenging due to anatomical, procedural or valve-related factors12. This challenge is further augmented during valve-in-valve (ViV) procedures due to the additional presence of transcatheter or surgical valve frames and leaflets3. Dedicated valve-specific cannulation techniques are required for operators to achieve coronary access, particularly in challenging scenarios24. To date, specific coronary cannulation techniques have only been described for the CoreValve/Evolut (Medtronic) and SAPIEN (Edwards Lifesciences) valve platforms following native aortic valve TAVI24.
Therefore, we performed ex vivo simulations of coronary access in a computed tomography-derived patient-specific pulsatile flow ViV-TAVI model consisting of an ACURATE neo2 (Boston Scientific) valve implanted inside a Carpentier-Edwards Perimount 25 mm surgical bioprosthesis (Edwards Lifesciences).
A challenging cannulation was simulated by selecting a patient with low coronary heights and narrow aortic sinus dimensions. An ACURATE neo2 valve was positioned at a high implantation depth and with severe commissural misalignment between the transcatheter and surgical valve posts (Supplementary Figure 1). Expert operators attempted to cannulate the left and right coronary arteries under fluoroscopic guidance using a wide range of differently sized and shaped catheters. The different cannulation approaches were visualised using an internally mounted borescope camera.
We describe three novel cannulation techniques, the vertical approach, narrow mammary and snake sinus, which all allow the obstructive elements of the ACURATE neo2 valve frame to be bypassed by a catheter (Figure 1, Supplementary Figure 2, Moving image 1- Moving image 3). These techniques are preferable in the setting of severe commissural misalignment and/or if the coronary ostia arise below the level of the upper crown adjacent to the pericardially covered stent frame. The vertical approach and internal mammary techniques are used for left and right coronary cannulation respectively, whilst the snake sinus technique can be used for cannulation of either ostium.
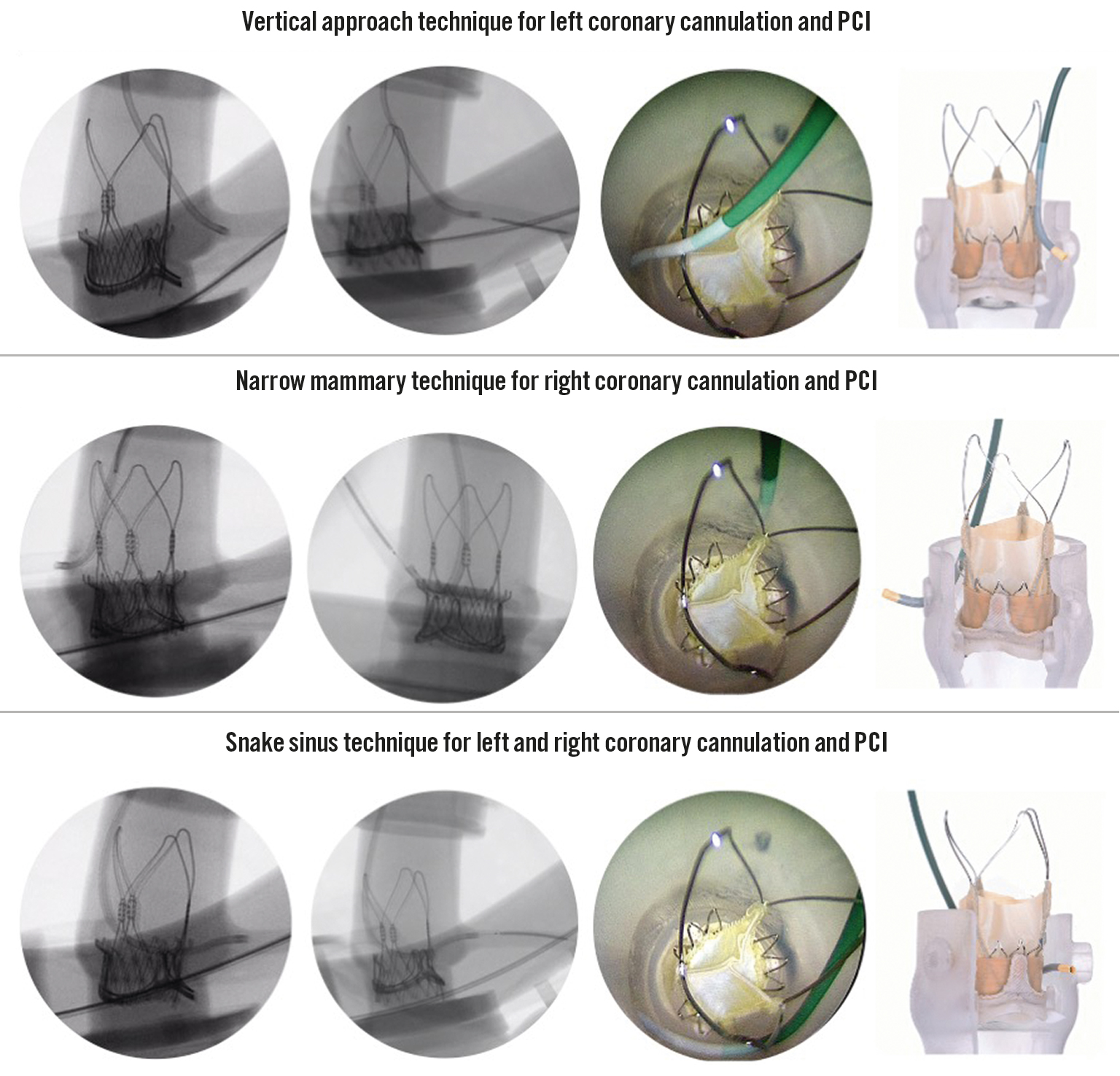
Figure 1. Novel coronary access techniques to bypass the ACURATE neo2 valve frame following ViV-TAVI. The vertical approach, narrow mammary and snake sinus techniques are presented with (from left to right): fluoroscopic images of diagnostic cannulation, percutaneous coronary intervention, internally mounted borescope view and ex vivo modelling. PCI: percutaneous coronary intervention; ViV-TAVI: valve-in-valve transcatheter aortic valve implantation
The reported techniques were reproduced by different operators using both 6 Fr diagnostic and guiding catheters. Adequate guiding catheter support was assessed for by simulating a percutaneous coronary intervention procedure. Delivery of an intracoronary wire, 3.0 non-compliant balloon and a 4.0x24 mm drug-eluting stent was feasible with all three techniques.
These techniques were specific to the ACURATE neo2 valve because of its unique split-level design with a short lower-stent frame and large open upper stabilisation arches. These techniques could not be replicated with the Evolut valve, due to the larger outflow portion of the valve, which leaves less room for a catheter to bypass the valve frame. All cannulations were performed from the femoral access route. Whilst the left radial access route may achieve similar results, we cannot comment on the feasibility of these cannulation techniques using the right radial approach, particularly in the presence of tortuous brachiocephalic anatomy. The safety and efficacy of these novel cannulation techniques has to be determined in different anatomical settings before further in vivo validation.
Acknowledgements
We would like to thank all members of the catheterisation lab simulation team at Boston Scientific R&D, Galway, Ireland.
Funding
We wish to declare that the equipment and materials for this study were provided and partially funded by a research grant from Boston Scientific. We confirm that Boston Scientific had no input into the planning, design or management of this study.
Conflict of interest statement
D. Dudek is on the advisory board for Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Ex vivo simulation of the vertical approach cannulation technique.
Moving image 2. Ex vivo simulation of the snake sinus cannulation technique.
Moving image 3. Ex vivo simulation of the narrow mammary cannulation technique.

