
CASE SUMMARY
BACKGROUND: An 80-year-old man with symptomatic severe aortic valve stenosis, haemodynamic instability, important comorbidities and a high surgical risk. Following bridge-balloon aortic valvuloplasty to recover from the unstable haemodynamics he was deemed suitable for transcatheter aortic valve implantation (TAVI) via the femoral access route.
INVESTIGATION: Fluoroscopy.
DIAGNOSIS: During TAVI, while the operators were preparing for prosthesis deployment (Edwards SAPIEN 3), the extra-stiff guidewire was accidentally withdrawn from the left ventricle (LV) to the ascending aorta.
MANAGEMENT: Valve orifice was re-crossed from the contralateral access, a snare was introduced through the AL1 catheter and pushed anterogradely through the valve. The extra-stiff wire was then captured in the ascending aorta and pulled back into the LV to complete the procedure.
KEYWORDS: balloon aortic valvuloplasy, complications, transcatheter aortic valve implantation (TAVI)
PRESENTATION OF THE CASE
An 80-year-old man with a history of previous inferior myocardial infarction and quadruple coronary artery bypass graft (CABG) was referred to our institution for severe aortic valve stenosis (AS) and heart failure in New York Heart Association (NYHA) Class IV. Hypertension, hypercholesterolaemia, familial history of ischaemic heart disease and overweight (body mass index=27 kg/m2) were his risk factors. A cardioverter defibrillator had been implanted four months before following an episode of syncope due to a sustained ventricular tachycardia. Relevant comorbidities included moderate chronic renal failure (glomerular filtration rate 37 ml/min), pulmonary emphysema and fibrotic interstitial disease, previous transient ischaemic attack and vascular encephalopathy, and Horton’s disease. The logistic EuroSCORE was 14%.
Transthoracic echocardiography showed a left ventricle with mild/moderate wall hypertrophy, normal telediastolic volume (58 ml/m2) and global systolic function (EF 60%), but inferior and posterior akinesia with wall thinning. The Doppler study measured a transaortic valve maximum gradient of 55 mmHg, mean gradient 30 mmHg. Aortic valve area (AVA) estimated by continuity equation was 0.85 cm2, indexed 0.4 cm2/m2.
Because of haemodynamic instability, the patient underwent balloon aortic valvuloplasty with reduction of the mean gradient from 41 to 21 mmHg. Coronary angiography showed patent grafts on chronically occluded native vessels. The patient recovered from the critical status and the clinical case was discussed again in a Heart Team meeting. He was deemed at prohibitive surgical risk but considered suitable for transcatheter aortic valve implantation (TAVI) via the left femoral access route.
Implantation of a 26 mm Edwards SAPIEN 3 prosthesis (Edwards Lifesciences, Irvine, CA, USA) under moderate sedation was planned through a Commander Delivery System™ including a 14 Fr eSheath™ (Edwards Lifesciences). A 6 Fr pigtail catheter was positioned in the ascending aorta after right femoral artery puncture. A temporary pacemaker lead was placed in the right ventricle via femoral vein access for rapid pacing during release of the bioprosthesis. The aortic valve orifice was crossed with a straight 0.035’’ wire inside an AL1 catheter. This wire was exchanged for a 0.038’’ extra-stiff wire with a broad handmade bend at the distal end lying into the left ventricle chamber. The delivery system with the crimped valve was inserted over that wire into the descending aorta and the distal deflated balloon was retrieved inside the valve to prepare for the inflation of the device. However, during the “fine alignment” consisting of the pullback of the balloon catheter until the prosthesis passed over the centre marker, the whole system slipped back and the extra-stiff wire came out of the left ventricle (LV) (Figure 1).
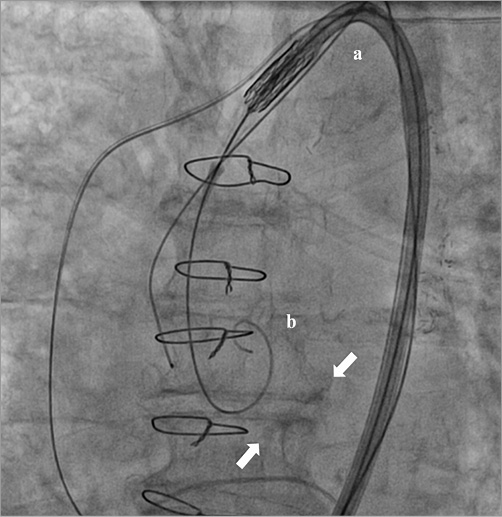
Figure 1. Guidewire shift above the aortic valve. The delivery system (a) and the extra-stiff wire (b) shifted above the aortic valve plane (arrows).
We were therefore stuck with the release system, trying to figure out how to re-cross the stenotic valve orifice and complete the procedure.
How would I treat?
THE INVITED EXPERT’S OP INION

The procedure was unexpectedly stopped due to a slippage of the stiff wire from the left ventricle. The SAPIEN 3 delivery catheter with the valve already on a balloon could not be pulled out and removed from the patient’s vasculature through the 14 Fr eSheath. There are two possible solutions in this situation:
1) The operators can advance the delivery system over the stiff and curved guidewire until it reaches the valve plane, then change this guidewire for a straight standard or hydrophilic 0.035” wire and try to cross the valve using the flex wheel of the delivery system handle to align the nose cone of the delivery system with the aortic orifice.
2) If the operators cannot accomplish this (which is likely to happen), they should pull back the valve and position it at the top of the eSheath or even try to withdraw the valve into the eSheath. They should then remove the valve/sheath assembly (important: you cannot leave the eSheath inside the femoral artery when you have to remove a SAPIEN valve!), leaving only a stiff guidewire in the femoral artery and aorta. Over this guidewire they should insert a new 14 Fr eSheath (sometimes a 16 Fr sheath is needed to prevent peri-sheath bleeding), through this sheath insert a second device with a new SAPIEN 3 valve and then complete the implantation. They should not reuse the recovered valve.
The slippage of the stiff wire from the LV cavity when the SAPIEN valve is already in the patient’s vasculature is a rare but troublesome complication of TAVI. The best solution is to retry crossing the aortic valve with a straight wire in order to accomplish the procedure.
Conflict of interest statement
A. Witkowski receives speaker’s fees from Edwards Lifesciences.
How would I treat?
THE INVITED EXPERTS’ OPINION

Over the years, transfemoral TAVI has progressively evolved, and complication rates have markedly decreased with continuously adjusted technological improvements and procedural refinements. However, although TAVI is now performed in a straightforward, standardised fashion, the procedure remains complex, especially if unanticipated events occur.
One fundamental principle of a standardised transfemoral TAVI procedure is the safe placement of an atraumatically shaped, long, stiff guidewire in the left ventricular apex. Once precisely positioned, the location of this guidewire needs to be carefully observed in order to prevent ventricular perforation by uncontrolled forward movement or accidental dislodgement into the ascending aorta.
Dall’Ara et al describe the dislodgement of the guidewire during alignment of the balloon-expandable Edwards SAPIEN 3 prosthesis resulting in a “deadlock” of the valve and delivery system. Fortunately, this unanticipated event does not result in an acute emergency situation with haemodynamic compromise, and there remains time to consider the options and carefully balance the decision making.
In the case of the self-expanding Medtronic CoreValve® prosthesis (Medtronic, Minneapolis, MN, USA), dislodgement of the guidewire is solved by pulling out the delivery system with the loaded prosthesis, re-crossing the stenosed aortic valve in a standard fashion and repositioning the guidewire in the left ventricular apex. The delivery device with the loaded prosthesis can then be reintroduced and the procedure completed as intended. This strategy apparently requires some additional procedural time, but no extensive and potentially harmful manoeuvres within the aorta, the aortic valve or the left ventricle.
Such a retreat can also be performed with the Edwards SAPIEN 3 prosthesis, if the access vessels are not extremely tortuous and calcified. Even after alignment of the crimped stent valve onto the balloon catheter, it usually remains feasible to salvage the aligned prosthesis into the eSheath and retrieve sheath and delivery system with the contained valve. However, sheath, delivery system and valve cannot be reintroduced, mandating use of a whole new kit.
The technological design of the system entails features that may be used to re-cross the aortic valve and reposition the stiff guidewire over the delivery system. The steerable flex catheter allows dual articulation for atraumatic passage of the aortic arch and for coaxial positioning of the stent valve to the aortic annulus. Additionally, fine adjustments towards the aorta or left ventricle can be made once the flex catheter has been retracted from the balloon. In order to re-cross the aortic valve, the delivery system may be steered atraumatically towards the aortic valve over the dislodged wire. The wire may then be exchanged for a hydrophilic long guidewire for valve crossing, which is facilitated by the articulation properties of the flex catheter. Once the guidewire has crossed the aortic valve, the tip of the balloon catheter may be carefully advanced into the left ventricle. In order to do so atraumatically, the flex catheter may be retracted from the balloon and the fine positioning features may be used. The guidewire may then be exchanged for a new stiff guidewire and the procedure completed. However, it must be borne in mind that this approach requires additional manipulations that need to be limited to avoid harmful complications such as stroke.
It should also not be forgotten that a TAVI prosthesis can be implanted in the aorta, if any other attempt at repositioning or salvage fails, a lesson that was learned in the early days with the CoreValve prosthesis1,2.
Conflict of interest statement
P. Kahlert is a clinical proctor for Edwards Lifesciences Inc. T. Rassaf is a clinical proctor for Medtronic. The other authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Possible options included:
a) pushing the whole cumbersome system towards the aortic valve to engage its orifice, knowing that in case of AS even a flexible and smooth wire may enter the LV with difficulty;
b) leaving the delivery system in situ, exchanging the extra-stiff wire for a hydrophilic or straight wire to approach the valve orifice;
c) retrieving the whole delivery system as well as the crimped valve through the eSheath, considering the possible difficulties due to the peculiar design of the sheath and that the manufacturer discourages re-use of the prosthesis, the eSheath or delivery system once they are retrieved, implying abortion or restart of the procedure from the beginning3.
We first tried to push the extra-stiff wire through the aortic valve orifice, but this manoeuvre was unsuccessful. No attempts to exchange the extra-stiff wire for a hydrophilic coated wire were made.
The delivery system was left in place as well as the extra-stiff wire. After removal of the contralateral pigtail catheter from the right femoral artery sheath, the AL1 catheter and the straight 0.035’’ wire were reinserted into the vascular bed to re-cross the aortic valve. Once inside the left ventricle, the AL1 was pushed deep into the apex to let its tip bend towards the LV outflow tract. The wire was exchanged for a 25 mm AndraSnare® (Andramed GmbH, Reutlingen, Germany) pushed anterogradely through the valve into the ascending aorta, where it could catch and grasp the loop at the distal end of the extra-stiff wire (Figure 2, Moving image 1). Then we were able to pull the extra-stiff wire into the LV cavity through the aortic valve orifice (Figure 3), allowing us to overcome the impasse and restart the manoeuvres to release the prosthesis.
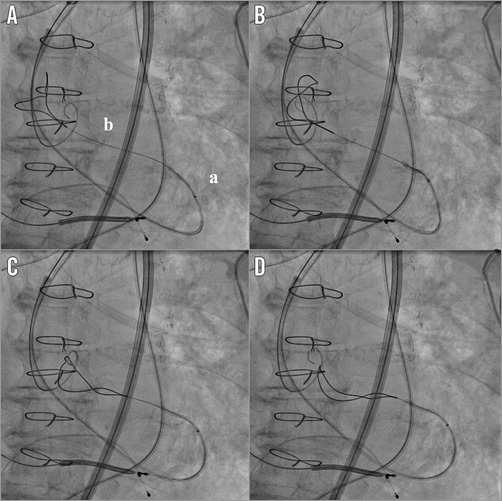
Figure 2. The rescue manoeuvre. AL1 catheter pushed deep into the LV with its tip directed towards the LV outflow tract (a). A snare (b) inserted within the catheter crossed the valve anterogradely and reached the ascending aorta where, after some attempts (from A to D), we were able to catch the extra-stiff wire tail (Moving image 1).
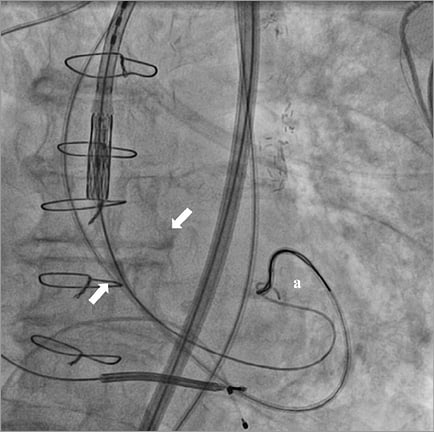
Figure 3. Guidewire capture with the snare. After grasping the extra-stiff wire (a), the snare could pull it inside the LV through the aortic valve orifice (arrows).
We were able to proceed with the prosthesis deployment with a relatively short waste of time, no risk of device damage during withdrawal, with an excellent final result and mild paravalvular leak (Figure 4, Moving image 2, Moving image 3).
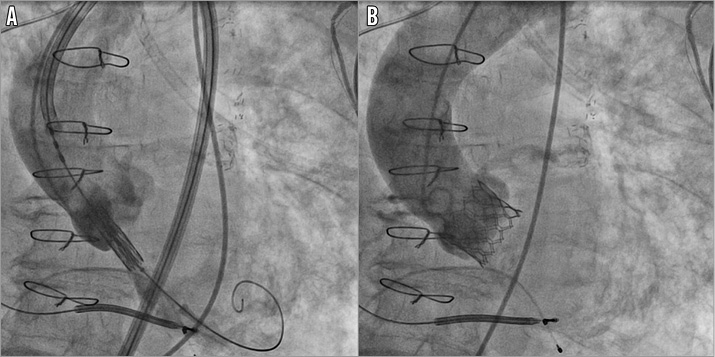
Figure 4. TAVI procedure. The delivery system properly placed through the aortic valve orifice and ready to proceed with the prosthesis release (A) (Moving image 2) and final angiography after deployment (B) (Moving image 3).
Conclusions
This case shows how a simple trick can overcome a potential deadlock during a TAVI procedure, avoiding the risks of retrieving the whole delivery system and bioprosthesis through the introducer sheath. Retrieval through the eSheath, despite being feasible based on preclinical testing, carries some potential risks such as prosthesis damage or vascular access complications. In fact, the eSheath increases in diameter when the delivery system and prosthesis are retracted, with a radial stress force on the vessel wall which, although low, might increase the risk of vessel injury. On the other hand, a retrieved prosthesis should not be re-used according to the manufacturer’s recommendations3.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. AL1 catheter pushed deep into the LV with its tip directed towards the LV outflow tract. A snare inserted within the catheter crossed the valve anterogradely and reached the ascending aorta where, after some attempts, we were able to catch the extra-stiff wire tail.
Moving image 2. The delivery system properly placed through the aortic valve orifice and ready to proceed with the prosthesis release.
Moving image 3. Final angiography after deployment.
Supplementary data
To read the full content of this article, please download the PDF.
AL1 catheter pushed deep into the LV with its tip directed towards the LV outflow tract. A snare inserted within the catheter crossed the valve anterogradely and reached the ascending aorta where, after some attempts, we were able to catch the extrastiff wire tail.
The delivery system properly placed through the aortic valve orifice and ready to proceed with the prosthesis release.
Final angiography after deployment

