
Abstract
Aims: The aim of this study was to evaluate vascular complications using the “parallel suture technique” in patients receiving an Edwards SAPIEN XT (SXT) or SAPIEN S3 (S3) transcatheter heart valve (THV).
Methods and results: Two hundred consecutive patients with symptomatic severe aortic stenosis treated with TF-TAVI were included in this study where the “parallel suture technique” was applied for vascular access-site closure. This was achieved by placing the sutures medial and lateral to the puncture site. Vascular access-site complications were defined as vascular dissection, perforation, obstruction, arteriovenous fistula or pseudoaneurysms, and classified according to the Valve Academic Research Consortium-2 (VARC-2) criteria. Duplex sonography was performed routinely in every patient. In patients receiving the S3, the sheath to femoral and iliac artery ratio was significantly lower than in the SXT group, reflecting reduction in sheath sizes for S3. More endovascular interventions were required after SXT implantation as compared to S3 (4% versus 1%, p=0.02). This was due to vascular obstruction or device failure. Moreover, increased life-threatening, major bleedings, and pseudoaneurysms were found in the SXT group (6% versus 1%, p=0.06, 13% versus 3%, p=0.009, 7% versus 1%, p=0.03, respectively).
Conclusions: The “parallel suture technique” using the ProGlide is associated with a low number of vascular complications, even when using larger sheath sizes.
Abbreviations
AS: aortic stenosis
AV: arteriovenous
BARC: Bleeding Academic Research Consortium
S3: Edwards SAPIEN S3 valve
SXT: Edwards SAPIEN XT valve
TAVI: transcatheter aortic valve implantation
TF: transfemoral
THV: transcatheter heart valve
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) is an alternative to surgical aortic valve replacement (SAVR) in high- and intermediate-risk patients with symptomatic severe aortic stenosis1-3. Advances in assessment, sheath and valve design, implantation techniques and periprocedural care have led to a significant improvement in the clinical outcomes of patients undergoing TAVI. The transfemoral (TF) approach is associated with superior outcomes as compared to other access routes4. However, procedure-related complications, acute kidney failure, stroke and most importantly vascular complications are associated with a significant increase in mortality and morbidity5. Among different vascular closure devices, ProGlide® (Abbott Vascular, Santa Clara, CA, USA) has been proven to be superior to Prostar® (Abbott Vascular)6. Previous studies have described a technique with two ProGlides rotated and deployed at the 10 and 2 o’clock position (“Perclose” method)7. Interference of both sutures may contribute to device failure and promote stenosis. Here we describe the efficacy of the “parallel suture technique”, a novel technique using the ProGlide in patients receiving Edwards SAPIEN XT (SXT) or SAPIEN S3 (S3) (Edwards Lifesciences, Irvine, CA, USA).
Methods
PATIENT SELECTION
We retrospectively evaluated the procedural and clinical outcomes of 100 consecutive patients with severe, symptomatic aortic stenosis (AS) who underwent TF-TAVI with SXT valves (from February 2013 until February 2014) compared to 100 consecutive patients treated with TF-TAVI using S3 valves (from February 2014 until September 2014). All patients were treated with two ProGlide sutures using the “parallel suture technique.” Duplex sonography of the access vessel and contralateral vessel was performed routinely in every patient within three days after the procedure independently of clinical symptoms.
The primary endpoints were major and minor vascular complications that were classified according to the Valve Academic Research Consortium-2 (VARC-2) criteria8. VARC-2 major complications were defined as access-site or access-related vascular injury (dissection, stenosis, perforation, rupture, arteriovenous [AV] fistula, pseudoaneurysm, haematoma, irreversible nerve injury, compartment syndrome, percutaneous device failure) leading to death, life-threatening or major bleedings, visceral ischaemia or neurological impairment. VARC-2 minor complications were defined as access-site or access-related vascular injury (dissection, stenosis, perforation, rupture, AV fistula, pseudoaneurysm, haematoma, irreversible nerve injury, compartment syndrome, percutaneous device failure) not leading to death, life-threatening or major bleedings, visceral ischaemia or neurological impairment. Secondary endpoints were haematoma, endovascular and surgical interventions.
Life-threatening bleeding was defined as fatal bleeding (BARC type 5) or bleeding into a critical organ (BARC type 3b and 3c) or bleeding causing hypovolaemic shock requiring surgery or vasopressors (BARC type 3b), bleeding with a drop in haemoglobin >5 g/dl or blood cell transfusions >4 units (BARC type 3b). Major bleeding was defined as overt bleeding associated with a drop in haemoglobin >3 g/dl or requiring transfusion of two to three units of red blood cells, or causing hospitalisation or permanent injury, or requiring surgery and not meeting the criteria for life-threatening bleeding. Minor bleeding was defined as any bleeding worthy of clinical mention that did not qualify as life-threatening or major (BARC type 2 or 3a)9. Calcifications were scored by CT scan as follows: score zero described no calcification, score 1 calcified spots, score 2 calcifications that extended over more than half of the vessel circumference, and score 3 described circular calcifications.
PARALLEL SUTURE TECHNIQUE WITH PROGLIDE
Multislice computed tomography guidance was used for vascular access-site planning. A puncture site above the femoral bifurcation and below the inguinal ligament without calcification of the anterior vessel wall was chosen (Figure 1A, Figure 1B). Using the femoral head as an anatomic marker (proximal, mid or distal), the puncture was carried out under fluoroscopy guidance in a posterior anterior projection (Figure 1C, Figure 1D). The puncture of the vessel was achieved at a 45° angle (Figure 2). In contrast to the standard “Perclose” method where the sutures are deployed after rotation, we deployed the sutures after moving them medially and laterally (Figure 3), thereby placing the two ProGlide sutures parallel to the vessel on both sides of the puncture site before insertion of the implantation sheath. Using an ex vivo model (VIABAHN® endoprosthesis; Gore Medical, Flagstaff, AZ, USA), we implanted two ProGlide sutures using the parallel technique (Figure 4A), inserted a sheath (Figure 4B), pulled out the sheath (Figure 4C) and pushed down the knots of the placed ProGlide (Figure 4D, Figure 4E). This resulted in a parallel orientation of the sutures, as shown in the view from the inner endoprosthesis (Figure 4F). In an ex vivo model we also compared both techniques. Orientation of both sutures after implantation using the parallel technique is shown in Figure 5A, using the standard technique in Figure 5B. After closure from the inside of the ex vivo graft, the parallel orientation of the sutures using the parallel suture technique (Figure 5C) can clearly be distinguished from the standard technique where the sutures are crossed (Figure 5D). Application of the sutures using the parallel technique in vivo is demonstated in a video where we also show the comparison with the ex vivo model using small inserts (Moving image 1). After valve implantation, the sheath is withdrawn while keeping the guidewire in place. The suture knots of both ProGlide devices are pushed down onto the vessel wall and tightened with the ProGlide knot pusher. Once there is satisfactory haemostasis the guidewire is withdrawn. If necessary, a third ProGlide suture is placed between the first two sutures. Access-site haemostasis and intact perfusion are confirmed by angiography.
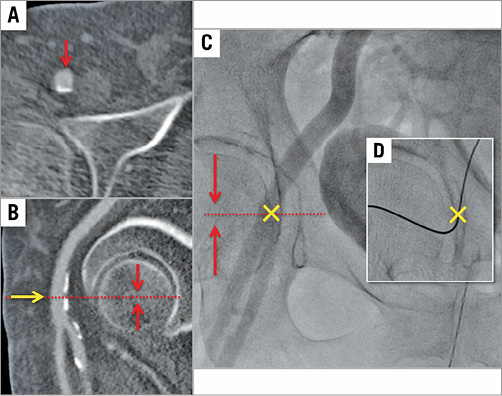
Figure 1. Vascular access puncture site. Identification of the optimal puncture site by multislice computed tomography (A & B) and fluoroscopy (C). Panel D shows the puncture site with the wire in place.
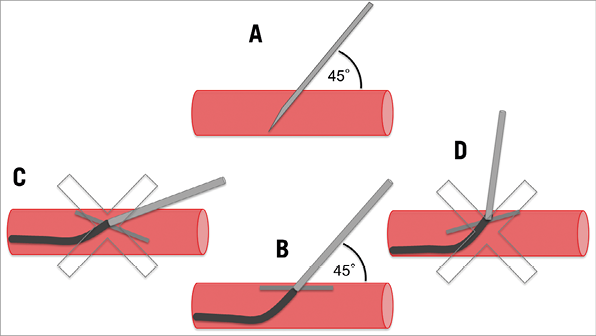
Figure 2. Vascular access puncture technique. Puncture and ProGlide should be placed at a 45° angle to the vessel wall : correct puncture (A), correct sheath insertion (B), flat-angle puncture (C) and high-angle puncture (D).
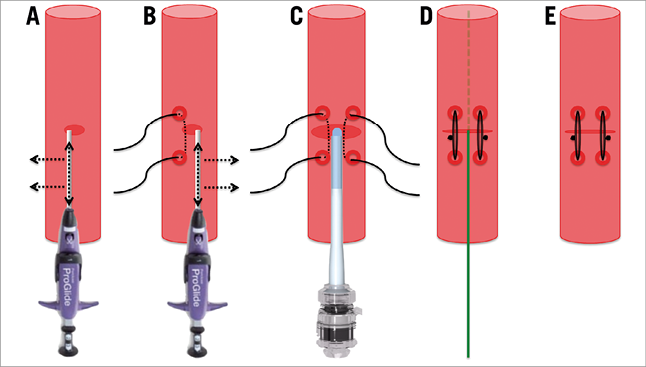
Figure 3. Vascular access ProGlide parallel suture technique. Placement of the ProGlide sutures was accomplished prior to insertion of the implantation sheath parallel to the vessel on either side of the arteriotomy. The first suture was placed medial (A) and the second suture lateral (B) to the puncture site without rotating the ProGlide device. After valve implantation, the sheath was withdrawn keeping the guidewire in place (C). The nodes of both ProGlide devices were pulled down onto the vessel wall and tightened using the pusher (D). After adequate haemostasis the guidewire was withdrawn (E).
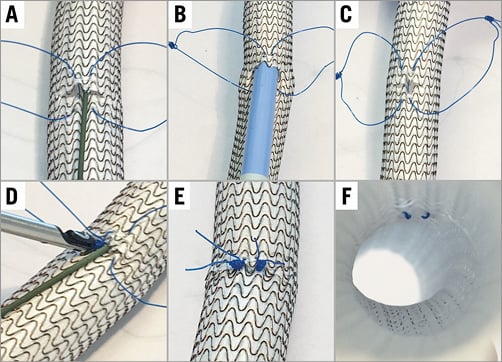
Figure 4. Closed arteriotomy using the parallel suture technique ex vivo. Two ProGlide sutures were inserted into a VIABAHN endoprosthesis ex vivo (A), a sheath inserted (B), pulled out (C), and the ProGlides tightened (D,E). Parallel orientation of the sutures from the intravascular view (F).
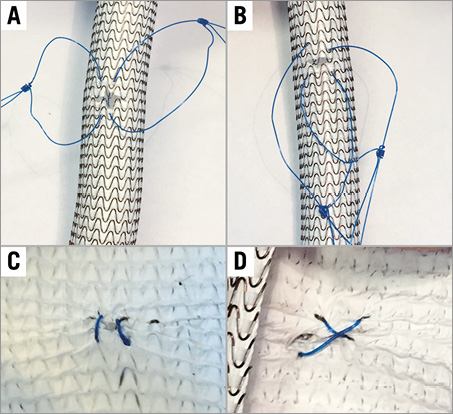
Figure 5. Comparison of the parallel suture technique with the standard technique using an ex vivo model. Two ProGlide sutures were inserted into a VIABAHN endoprosthesis using the parallel suture technique (A) or the standard technique (B). After tightening the knots, parallel (C) or crosswise (D) orientation of the sutures from the intravascular view is shown.
MANAGEMENT OF ANTICOAGULATION
During the procedure, patients were under full anticoagulation (ACT >250 s) with heparin. After the procedure and before sheath removal, patients without an indication for anticoagulation were fully antagonised with protamine, whereas patients with an indication got only half the dosage.
TREATMENT OF VASCULAR COMPLICATIONS
Management of vascular complications was left to the operator’s discretion. Dissections and stenosis were preferably treated with percutaneous transluminal angioplasty (PTA), and perforations by implantation of a covered stent.
STATISTICAL ANALYSIS
Categorical data are presented as counts or proportions (%). Continuous data are presented as mean±standard deviation. Differences between the groups were assessed using the χ2 or Fisher’s exact test for categorical data and the t-test for continuous data, while skewed distributions were compared using the Mann-Whitney non-parametric test. We used SPSS, Version 22 (IBM Corp., Armonk, NY, USA) for the statistical analyses.
Results
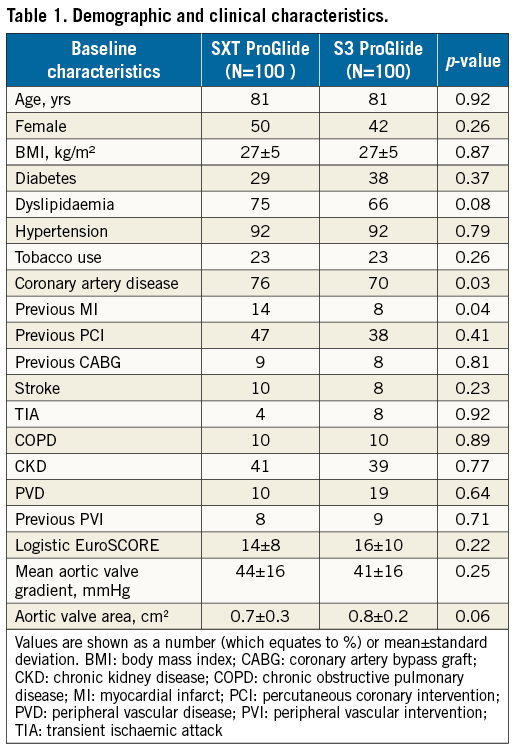
BASELINE CHARACTERISTICS
The baseline characteristics of the study population are shown in Table 1. The overall patient population was intermediate to high risk (logistic EuroSCORE 14±8 and 16±10, respectively, in the SXT and S3 groups, p=0.22). Compared to patients in the S3 group, patients in the SXT group had a higher rate of previous myocardial infarction (MI) and percutaneous coronary interventions (PCI), whereas a higher proportion of patients receiving the S3 valve had a diagnosis of peripheral vascular disease (PVD).
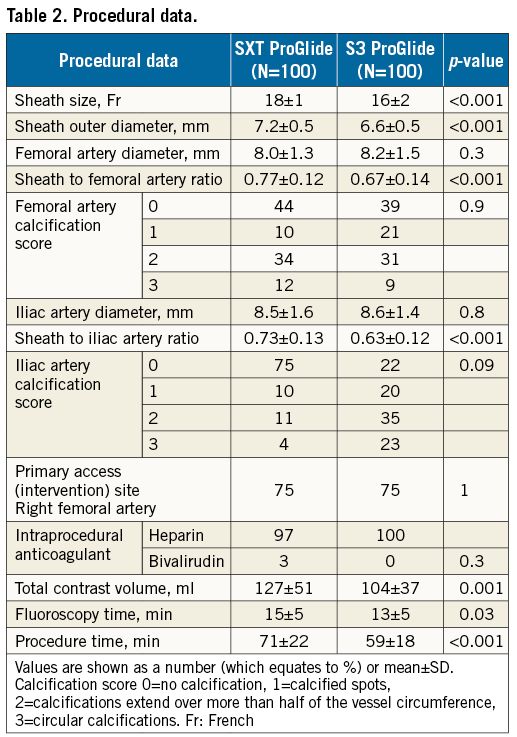
PROCEDURAL DATA
Procedural data are shown in Table 2. Common femoral artery size was similar in both groups. Sheath size was 16-20 Fr in the SXT group and 14-16 Fr in the S3 group. The outer diameter of the sheath was smaller in the S3 group due to the decrease in sheath size. The smaller sheath to iliac and femoral artery ratio in the S3 group reflects similar vessel sizes in both groups (Table 2). In the SXT group, 4% of access vessels were <6 mm compared to 7% in the S3 group. The total procedural contrast volume, X-ray time and overall procedure time were also lower in the S3 group as compared to the SXT group.
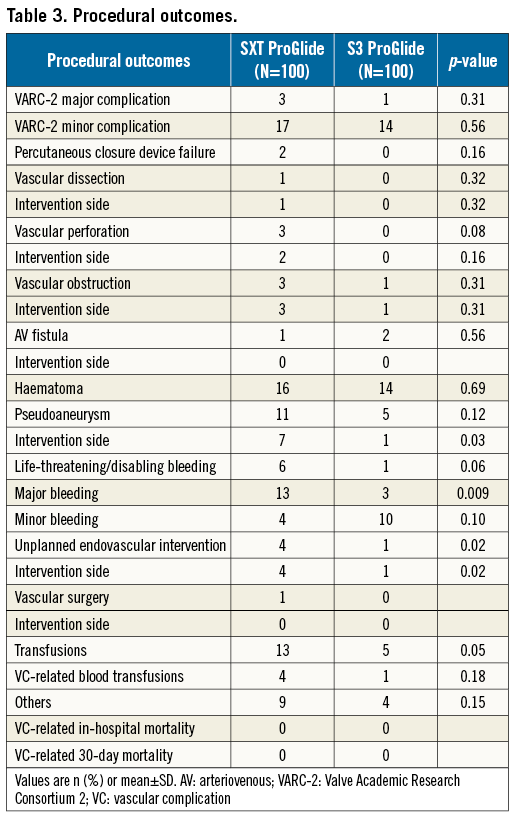
PROCEDURAL OUTCOMES
Procedural outcomes are shown in Table 3. Valve implantation was successful in all procedures. No patient died during the hospital stay or within 30 days. One patient in the SXT group suffered from minor stroke during hospital stay (modified Rankin score <2 after 30 days). In the SXT group, three VARC-2 major complications were observed: two patients underwent endovascular implantation of a covered stent for common femoral artery bleeding, and one patient was treated surgically for perforation of the common carotid artery after attempted central line placement. VARC-2 minor complications included two obstructions of the common femoral artery (one due to dissection that was treated by PTA, and one requiring stent implantation), one common femoral artery stenosis that was treated conservatively, and seven access-site pseudoaneurysms: two were treated by thrombin injection, four compressed manually and one spontaneously thrombosed. In addition, 18 access-site haematomas >5 cm were observed.
In the S3 group, one VARC-2 major complication was observed due to retroperitoneal bleeding requiring transfusion that was managed conservatively. Sixteen VARC-2 minor complications were observed, including one vascular obstruction of the common femoral artery that was treated by PTA, 15 access-site haematomas >5 cm, and one pseudoaneurysm treated by thrombin injection. Comparison of the two groups revealed that unplanned vascular interventions were significantly more frequent in patients after SXT implantation as compared to S3 implantation (4% versus 1%, p=0.02).
Six life-threatening bleedings occurred in the SXT group. These comprised four pericardial effusions, treated by pericardial puncture, one failed vascular closure of the common femoral artery that was treated by covered stents, and one perforation of the carotid artery that required surgical treatment. With S3 implantation, only one case of life-threatening bleeding occurred due to haemorrhagic pericardial effusion that was treated by pericardial puncture.
There was a trend towards higher major bleeding in the SXT group compared with the S3 group (14% versus 5%, respectively, p=0.09). Minor bleedings were observed in 4% of patients after SXT compared with 11% after S3 implantation (p=0.18). The transfusion rate was comparable in both groups (Table 3).
Discussion
TAVI is rapidly evolving to become the standard of care for patients with severe AS at high and intermediate risk for surgical aortic valve replacement. Vascular complications are an important cause of morbidity and mortality and thus remain an important determinant of TAVI outcomes. Our use of the “parallel suture technique” for the vascular access site demonstrated a low rate of major and minor vascular complications. Major vascular complications as defined according to the VARC-2 guidelines reported in other TAVI series ranged from 9.8% up to 51.6%, with an associated twofold to threefold increase in 30-day mortality10. With the use of the “parallel suture technique,” we observed a VARC-2 major vascular complication rate of less than 3% after TF-TAVI. This is a significant decrease when compared to a recent meta-analysis that showed a major vascular complications rate of 11.9%10; however, minor VARC-2 complications occurred more frequently in our study (up to 18%) when compared to what was reported in the meta-analysis (9.7%). This can be explained by the fact that we performed ultrasound on every patient prior to discharge to monitor for vascular complications, probably elevating the rate of diagnosis of minor complications above that which may have been picked up by clinical evaluation alone, as performed in previous studies. Thus, we were able to identify even small pseudoaneurysms that might thrombose spontaneously. This may explain our relatively high rate of pseudoaneurysms. Consistent with other studies, we found increased bleeding and transfusions in patients suffering from vascular complications. However, this was not associated with an increase in mortality, probably due to the low number of major vascular complications.
Studies comparing different suture devices have reported conflicting results, one favouring Prostar over ProGlide11, another favouring double Prostar over single Prostar12, another favouring ProGlide over Prostar6. This underlines the importance of the use of an optimal deployment technique. We report a 3% VARC-2 complication rate and no requirement for access-site surgery with the parallel suture technique in patients undergoing a TAVR procedure. These data appear to be better than those recently reported with the standard “Perclose” technique: a 7.5% VARC-2 major complication rate and an incidence of 2.9% of access-site surgery13. Our data suggest that vascular complications are low when using the parallel suture technique with two different valve types and a range of access sheath sizes from 14-20 Fr, although superiority of this technique can only be determined by randomised studies.
Several studies have described female sex and sheath size as independent risk factors for vascular complications after TAVI5,10,14. We did not find a reduction in vascular complications after S3 as compared to SXT implantation; this could be attributed to the ability to perform TF-TAVI in more patients with peripheral vascular disease due to the reduction in the sheath size of the S3 THV. In contrast to other studies using various devices and techniques for vascular access-site closure, this is the first comprehensive report of clinical outcomes using the parallel suture technique with the use of two ProGlide devices. Similar to the suture techniques of vascular surgeons, we place two sutures parallel to the axis of the vessel, minimising the risk of stenosis due to oblique foreshortening of the vessel diameter. In comparison to the more commonly used technique involving rotation of two ProGlide devices (“Preclose” method), this novel parallel suture technique may decrease the risk of interference with the sutures leading to subsequent device failure. Moreover, this technique allows direct placement of a third suture between the two previously placed sutures without interfering with them.
It is also important to note that all of our vascular access-related complications after TAVI were successfully treated using an interventional approach, further underlying the safety of this approach.
The longer procedure times in patients receiving SXT may reflect the longer compression times required to achieve complete haemostasis on account of the larger sheath size. This is supported by the fact that all patients underwent a similar protocol of full reversal of heparin with protamine and manual compression until complete haemostasis was achieved.
The low frequency of access-site complications and the lack of any cases of surgical repair after TF-TAVI using the parallel suture technique with ProGlide suggest that vascular access and closure for TAVI can be performed without surgical supervision, supporting the concept of a minimalist approach to TAVI via the femoral artery. This report supports the use of this technique with other TF transcatheter valve systems regardless of sheath size.
Study limitations
This is a retrospective study conducted at a single centre with a small sample size. The inherent limitations of a non-randomised study apply, including selection bias and the lack of a control group.
Conclusions
The parallel suture technique using ProGlide is associated with a low number of vascular complications even when using larger sheath sizes as shown in both the SXT and S3 valve systems.
| Impact on daily practice The low frequency of access-site complications after TF-TAVI using the parallel suture technique with ProGlide suggests that vascular access and closure for TAVI can be performed without surgical supervision and supports the concept of a minimalist approach to TAVI via the femoral artery. This report supports the use of this technique with other TF transcatheter valve systems regardless of sheath size. |
Conflict of interest statement
A. Kasel is a medical consultant for and receives research support from Edwards Lifesciences. The other authors have no conflict of interest to disclose.
Supplementary data
Moving image 1. Parallel suture technique in vivo.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Parallel suture technique <i>in vivo</i>.

