Abstract
Aims: Vascular complications remain the main limitation of transfemoral aortic valve implantation. Based on a single-centre experience, we aim to detail the type, management and impact of those vascular complications.
Methods and results: From October 2006 to January 2009, 54 transfemoral aortic valve implantations were performed using the Edwards SAPIEN™ prosthesis. Nine patients (16.7%) developed vascular complications. Five patients (9.3%) had ruptures which necessitated a surgical bypass. Four patients (7.4%) had dissection necessitating repair using stenting in all four patients and associated bypass in two of them. Vascular complications led to death in one patient (1.9%), reintervention in one (1.9%), and transfusions in seven (13%). Five vascular complications occurred in the first 20 patients (25%), and only four in the last 34 (12%).
Conclusions: Vascular complications of transfemoral aortic valve implantation are frequent and seem to be influenced by experience. They are associated with a high need for transfusion and could lead to major events such as death or reintervention. These findings highlight the importance of a multidisciplinary approach for patient selection and management of the procedure.
Abbreviations
AS: aortic stenosis
AVR: aortic valve replacement
CT: computed tomography
ECG: electrocardiogram
EuroSCORE: European System for Cardiac Operative Risk Evaluation
STS-PROM: Society of Thoracic Surgeons Predicted Risk Of Mortality
TAVI: transcatheter aortic valve implantation
TEE: transesophageal echocardiography
TF-AVI: transfemoral aortic valve implantation
VCs: Vascular complications
Introduction
Transcatheter aortic valve implantation (TAVI) has expanded the treatment options for aortic stenosis (AS) in patients at high surgical risk for aortic valve replacement (AVR)1-6. Because of the large diameters of current devices, the risk of vascular complications (VCs) represents the main limitation of the transfemoral approach. However, no previous study specifically focused on this question. Our aims were to detail the VCs related to transfemoral aortic valve implantation (TF-AVI) using the Edwards SAPIEN prosthesis and their management, and to evaluate their impact on clinical outcome.
Methods
The decision for treating high-risk patients with AS was based on a multidisciplinary medico-surgical evaluation as previously described7,8. The transfemoral approach was considered as the first option, and transapical or retroperitoneal approaches only in case of contraindications.
Vascular screening
Femoro-iliac and aortic anatomy were studied with both computed tomography (CT) scan (Figure 1) and conventional angiography9 in all patients to assess minimal luminal diameters, vessel wall calcification and vessel angulation.
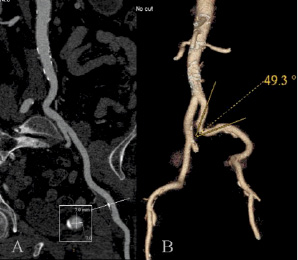
Figure 1. Curved view (a) and 3D VRT (b) reconstructions of the aorta and left ilio-femoral arteries. The arterial tree was rated grade 1 for calcification with a minimum lumen mean diameter on the common femoral artery of 7 mm and a minimum angle of 49° on the primitive iliac artery.
TF-AVI was deemed unsuitable in the following cases:
1. Previous aorto-femoral bypass,
2. Bulky aortic atherosclerosis (severe atheroma with mobile element > 5 mm),
3. Porcelain thoracic aorta (extensive and circumferential aortic calcification)7,
4. A minimal luminal diameter < 7 mm for the 22 Fr sheaths (external diameter 8.38 mm) and < 8 mm for the 24 Fr sheaths (external diameter 9.14 mm),
5. Severe vessel angulations (minimal angle < 40°),
6. Circumferential and extensive vascular calcification.
Parameters 1 to 4 were considered as formal contraindications whereas parameters 5 to 6 were considered along with diameter: the more severe the calcification or the vessel angulations, the larger the arterial diameter that was required.
A. Femoro-iliac Angiography (Axiom Sensis XP, Siemens AG, Munich, Germany) was performed at the same time as coronary angiography screening. Twenty ml of iodinated contrast was injected into the infra-renal aorta to study the left femoro-iliac axis, in a 20° right anterior oblique, 20° caudal view. Then, 10 ml was selectively injected in the right common iliac artery, in a 20° left anterior oblique, 20° caudal view.
B. Multislice CT was performed in a separate setting to coronarography in all patients and using medical preparation10 to avoid worsening of renal function. A 64-multidetectorCT scanner (LightspeedVCT, GEHealthcare Ltd, Little Chalfont, Buckinghamshire, UK) under prospective electrocardiogram gating with an axial field of view of 50 cm and a longitudinal coverage of the entire aorta and ilio-femoral arteries was used. Contrast enhancement was achieved with 90 ml of Iobitribol350 mg/ml (Xenetix®,Guerbet, Villepinte, France) injected at a rate of 3 ml per second. The thickness of reconstructed images was 0.625 mm.
Image analysis was performed using the GE software. The curved view was used to allow to: a) visually identify and measure the location of the smallest diameter on the left and right femoro-iliac arteries (Figure 1), b) measure the smallest diameter on a planperpendicularto vessel axis, and c) grade vessel wall calcification (grade 0: no calcification, grade 1: mild calcification, grade 2: severe or circumferential calcification).
C. A multidisciplinary discussion was finally held between cardiologists, radiologists and vascular surgeons to assess the feasibility and side of access site of the TF-AVI. When discrepancies between conventional angiography and MSCT were observed, preference was initially given to the former but, with further experience, MSCT was considered as the gold standard method for the quantitative assessment of femoroiliac diameters.
Procedure
TF-AVIs were performed under general anaesthesia using the Edwards SAPIEN™ prosthesis (Edwards Lifesciences Inc, Irvine, CA, USA). Patients received aspirin 75 mg once daily and clopidogrel 75 mg once daily for at least four days prior to the procedure, or a loading dose of clopidogrel 300 mg the day before. Heparin 70 UI/kg was given intravenously before crossing of the aortic valve.
A. Vascular access
Vascular access was performed using two different methods over time: a percutaneous X-ray guided puncture for the first 19 patients; then a surgical, view guided puncture for the last 35 patients was decided by medico surgical consensus to render the arterial repair easier.
X-ray guided puncture: the puncture was performed under fluoroscopic guidance overlying the upper part of the bony femoral head. A 6 Fr sheath by Terumo (Terumo Corp., Tokyo, Japan) was placed in the common femoral artery and, after stepwise dilatation with 8, 10 and 12 Fr dilatators, a 14 Fr sheath by Cook (Cook, Bloomington, IN, USA) was placed to allow balloon dilatation of the aortic valve. Thereafter, further stepwise dilatation with 16, 18, 20, 22 (± 24) Fr dilatators was performed on an Amplatz Extra Stiff 0.035 wire before introducing the 22 or 24 Fr sheath on the same wire. The sheath was pushed through the femoro-iliac axis to the aorta, using a gentle twisting motion of the catheter under careful fluoroscopic control.
View guided puncture (Figure 2): The common femoral artery was exposed and dissected free just below the inguinal ligament to gain access to a soft area of the artery.
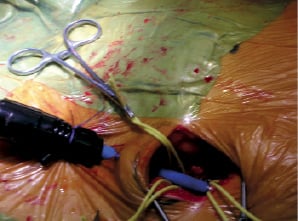
Figure 2. View guided puncture. Visual analysis and manual palpation allows puncture of the common femoral artery. The sheath is then passed through the skin to provide a firm anchor.
Proximal and distal control of the vessel was obtained with vascular loops. After inspection and manual palpation of the artery, a puncture was made through the skin and subcutaneous tissues 2 cm below the primary incision, providing a firm anchor for the sheath. The needle was then inserted in the anterior vessel wall avoiding bulky plaques. Then, the 22 or 24 Fr sheath was introduced on an Amplatz Extra Stiff wire or on an Extra Back Up Meier wire, under view control, without predilatation.
B. Valve implantation was performed through the flexible catheter RetroFlex™ (Edwards Lifesciences, Irvine, CA, USA) as previously described1-4,7.
C. Sheath withdrawal
After valve implantation, the sheath was withdrawn on the stiff wire to the upper part of the external iliac artery. An angiogram in an anteroposterior view was then performed to study the abdominal aorta, the common iliac, and the first two centimetres of the external iliac artery (Figure 3).
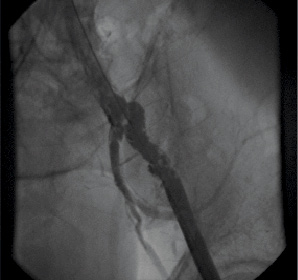
Figure 3. Angiogram in an anteroposterior view showing an extensive dissection of the common iliac and external iliac arteries.
Before complete withdrawal of the sheath, the access site and the visible portion of the external iliac artery were inspected to detect ruptures. In the absence of any VCs, the common femoral artery was clamped and repaired with polypropylene sutures. After complete surgical repair, a final angiogram was performed.
D. Post procedural care
Patients were then directed to the Intensive Care Unit for at least 48 hours. Physical examination was performed every six hours to detect signs of limb ischaemia. Antiplatelet therapy consisted of aspirin 75 mg daily lifelong and clopidogrel 75 mg daily up to three months. If continued oral anticoagulation was mandatory, only one antiplatelet agent was maintained. Inguinal drainage was withdrawn after 48 hours if blood loss was less than 50 cc/24h.
Mobilisation was usually authorised after 48 hours.
Definition of VCs
VCs were defined as complications directly related to prosthesis delivery:
– Vascular rupture: contrast media extravasation on angiogram or visual evidence of arterial wall disruption.
– Thrombotic complication: arterial filling defect related to thrombosis.
– Vascular dissection: radio lucent area within the vessel, requiring repair (Figure 3).
– Any access site complication requiring secondary surgical procedure.
Follow-up
In-hospital clinical and echocardiographic data were obtained before discharge. All clinical adverse events were prospectively recorded at 30 days, six months and yearly thereafter.
Statistical analysis
Quantitative variables were expressed as median and interquartile ranges [25th-75th percentiles]. Between-group comparisons used the Mann-Whitney U test for quantitative variables and the Fisher exact test for qualitative variables. The impact of VCs on outcome was evaluated with analysis of in-hospital death, the need for reintervention, transfusion and the length of stay. The effect of the learning curve on VCs was assessed by comparison of their occurrence between the first 20 and the last 34 patients.
Six-month mortality was analysed using the Kaplan-Meyer method and comparisons used the logrank test.
All tests were two-sided. A p-value <0.05 was considered significant. Statistical analysis was performed using statistical software Statistica version 5.0 (Statsoft Inc., Tulsa, OK, USA).
Results
Population
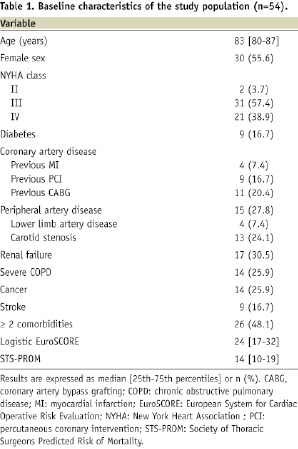
From October 2006 to January 2009, 188 high-risk patients were consecutively referred for the management of severe and symptomatic AS. Of these patients, 54 (29%) were treated with TF-AVI (Table 1) while 134 others (71%) were contra-indicated.
Contra-indications were related to general condition or too large aortic annulus in 71 cases (53%), or specifically to unfavourable femoro-iliac anatomy in 63 cases (47%). The contraindicated patients were treated by either transapical (n= 29, 21.6%) or retroperitoneal TAVI (n=2, 1.5%), conventional AVR (n=25, 18.7%), or medically (n=78, 58.2%). The percentage of patients rejected for TF-AVI remained stable over time. The first 20 patients treated using TF-AVI came from 73 screened patients, of whom 25 (34%) were rejected for vascular reasons. In the following 115 screened patients, 38 (33%) were rejected for vascular reasons (p=0.86).
Procedure
TF-AVI failed in five cases. Failure was directly related to vascular access in 3/5 cases. In the first case (case n°8), the minimal lumen diameter was 5.7 mm by CT scan and 7.2 mm by angiography. The artery presented severe calcifications. The iliac bifurcation could not be crossed by the 22 Fr sheath and the procedure was complicated by rupture of the external iliac artery requiring a bypass. In the two other cases (cases n°15 and 28), 22 Fr sheaths could not be introduced in the common femoral artery due to its diameter being to small at the access site (6.5 and 7.1 mm respectively as measured by CT scan and 7.1 and 6.9 mm by angiography). The procedures were aborted and the patients did not suffer any complications.
The two failures of non-vascular origin (cases n° 11 and 16 ) were due to the inability to cross the aortic valve with the prosthesis in a patient with severe kyphoscoliosis and to perforation of the left ventricle by the wire in another patient, leading to hemopericardium and intraprocedural death.
Vascular complications
Nine patients (16.7%) experienced VCs as defined previously (Table 2). Variables associated with the occurrence of these VCs are presented in Table 3.
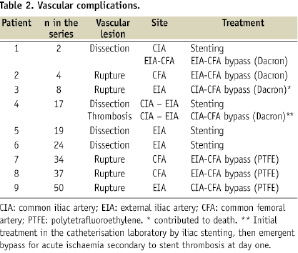
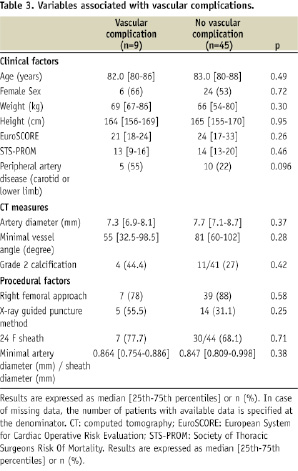
A. Type and location
Five patients (9.3%) had arterial rupture and four (7.4%) had dissections. Two other patients had localised dissections without flow abnormalities, which did not require any treatment and, thus, were not considered as VCs. There were neither any thrombotic complications, nor access site complications requiring secondary surgery. There were five VCs in 19 patients (26%) with X-ray guided puncture and 4 VCs in 35 patients (11%) with view guided puncture (p=0.25).
No VC involved the abdominal or thoracic aorta or induced haemodynamic instability. The common iliac artery was involved in 3/9 cases, the external iliac artery in 7/9 cases, and the common femoral artery in 4/9 cases, several locations being possible in one given patient. Arterial ruptures affected the common femoral artery or the distal external iliac artery and were visually detected prior to sheath withdrawal. No blood extravasation was seen. On the other hand, dissections were located more proximally (common or external iliac artery) and were detected by the final angiography.
B. Treatment
The five patients with vascular rupture were treated using surgical bypass (9.3%). Four patients (7.4%) with arterial dissection were managed using endovascular stenting for the management of arterial dissection (Advanta V12®, Atrium Medical Corporation, Hudson, NH, USA). Ilio-femoral bypass was associated in two patients: one was performed during the index procedure, the other one was emergently needed one day later because of acute limb ischaemia due to stent occlusion. This latter case was the only secondary surgical procedure.
C. Impact on outcome (Table 4)
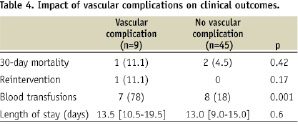
VCs contributed to in-hospital death in one patient who died from multi organ failure two days after vascular bypass. The need for transfusion was increased in patients with VCs, (all transfusions being secondary to blood loss induced by the TF-AVI) (Table 4). Overall, VCs were responsible for one death (1.9%), death or secondary vascular intervention in two patients (3.7%) and death or secondary intervention or transfusions in seven patients (13%). Both in-hospital death and reintervention occurred in the early experience (Table 5). There were no significant differences in hospital mortality or length of stay between patients with and without VCs.
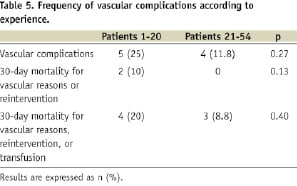
Median follow-up duration was 225 days (69-352). Six-month survival was 88.9±10.5% in the nine patients who had VCs and 89.2±5.2% in patients without VCs (p=0.71). No vascular complication occurred during follow-up in patients with or without VCs. No functional sequelae related to VCs were reported during follow-up.
Discussion
To our knowledge, this study is the first to analyse in detail VCs occurring during TF-AVI with the Edwards SAPIEN prosthesis using a standardised assessment. VCs occurred in nearly one fifth of the patients. They resulted in a higher rate of transfusions and could lead to major events such as death or reintervention.
Frequency
Complication rates should be interpreted according to the systematic search for all types of VCs in the present study. Previous reports did not focus specifically on VCs and definitions across the different studies were not standardised, which may have led to underestimation of their incidence, particularly for non major VCs2-6,11.
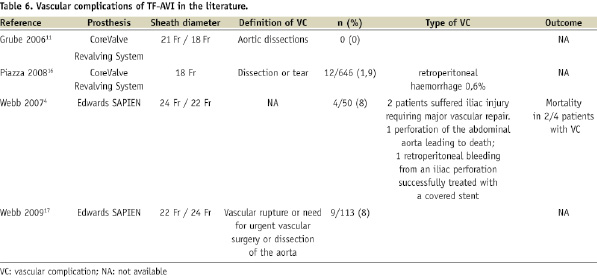
As expected, VCs appear to be less frequent with the Medtronic CoreValve System® (Medtronic, Minneapolis, MN, USA) than with the Edwards SAPIEN™ prosthesis (Table 6). This is likely to be due to the smaller sheath size (18 Fr for the current generation of the Medtronic CoreValve System®, compared to 22 or 24 Fr for the Edwards SAPIEN™).
Type and clinical consequences
Contrary to previous reports, we encountered neither aortic dissections nor ruptures. Most of our VCs consisted of femoral, iliac dissections or ruptures and could be managed during the index procedure. In our experience, severe complications were rare and only one VC led to in-hospital death at the beginning of our program. The most frequent impact of VCs was an increased rate of blood transfusion. There were no significant difference in length of stay and in-hospital mortality between patients with and without VCs. However, the small sample size limits the statistical power of these comparisons.
Treatment
When VCs occurred, two patients could be managed by isolated arterial stenting, but the others required surgical grafting. This observation and the unpredictable risk of major VCs during TF-AVI stress the need to be able to tackle any VC immediately with the availability of all the equipments (aortic occlusion balloon, peripheral covered and non-covered stents) and skills. In the present series, all VCs were instantaneously managed by vascular surgeons who were present in the catheterisation laboratory.
Prevention
Careful vascular screening is mandatory for all candidates to TF-AVI. Preference was initially given to conventional angiography. However, with growing experience, we now consider multislice CT as the reference method. Despite the absence of statistical significance, failure and VC rates as well as their consequences tended to be lower over time, which may be due to improved patient selection and management. This finding is consistent with other large series using the Edwards SAPIEN™ prosthesis. In comparison with the REVIVE and phase 1 REVIVAL experience, the more recent SOURCE Registry reported that, with further experience, incidence of VCs decreased and VCs were no longer associated with mortality.
Alternative approaches should be considered in poor, or borderline, candidates to TF-AVI.
The two main alternatives are the use of 18 Fr Medtronic CoreValve System® allowing implantation in patients with ilio-femoral arteries > 6 mm and the transapical approach with the Edwards SAPIEN prosthesis8,12. Other alternatives are the retroperitoneal13 approach with the Edwards SAPIEN™ prosthesis or the subclavian arterial approach14 with the Medtronic CoreValve System®.
Abortion of the procedure should be considered in case of major resistance or movement of arterial calcification on fluoroscopic control during sheath progression.
An unsolved issue for preventing VCs is the choice between percutaneous access with surgical repair, surgical exposure and repair, or pure percutaneous access and closure (in this approach, ultrasound-guided vascular access may provide further guidance15. Today, these different approaches are being used, but their respective risk/benefit ratios have not been compared.
In the near future, the availability of the 18 Fr sheath compatible Edwards TX prosthesis, which is currently under evaluation, should allow the prevention of a substantial proportion of these VCs.
Limitations
The definitions used for VCs may be considered somewhat arbitrary. This underlines the need for setting precise definitions for future evaluations of TF-AVI.
The small number of events precludes the identification of predictive factors with an adequate statistical power. However, the single-centre nature of the present study allowed a uniform and detailed assessment and management of all the patients and VCs.
Finally, this series is limited to the description of VCs related to the currently available Edwards SAPIEN™ prosthesis, using surgical repair of the femoral artery. However, the present description could be useful for comparison with other approaches and devices.
Conclusion
This specific analysis of VCs using standardised definitions shows that they occur frequently when using the Edwards SAPIEN prosthesis. VCs increased the need for transfusions and could lead to major events such as death or reintervention. This underlines the necessity of a careful screening and close collaborative management to reduce their frequency and clinical impact. In the near future, the reduction in the diameter of devices should help to lower the rate and the severity of VCs, and allow a higher number of patients to benefit from the TF approach for TAVI.

