Abstract
Aims: The aim of this study was to evaluate the safety and performance of a specifically designed, dedicated TAVI guidewire.
Methods and results: From May 2011 to August 2012, 39 consecutive patients referred for TAVI were prospectively enrolled in a first-in-man, open label, non-randomised feasibility study to evaluate the safety and performance of a specifically designed, dedicated TAVI guidewire in our institution (mean age 80.4±5.1 years, mean logistic EuroSCORE 26.8±11.7%, n=29 CoreValve transfemoral, n=8 CoreValve direct aortic, n=1 Edwards SAPIEN valve direct aortic, n=1 CoreValve subclavian). The primary safety endpoint was reached successfully with the dedicated TAVI guidewire in all 39 cases with no cases of guidewire displacement from the left ventricle during the procedure. In three cases the wire was repositioned to optimise loop position using a pigtail catheter prior to valve implantation. There were no cases of guidewire-related procedural complications. The mean delivery system tracking time using the guidewire was 1.4±0.6 minutes and the mean deployment time for TAVI was 13.8±7.8 minutes.
Conclusions: This represents the first recorded use of a dedicated TAVI guidewire to treat patients with a transcatheter aortic valve. The wire is easy to place, safe to adjust within the ventricle, and the stiffness of the wire facilitates valve tracking through tortuous anatomy. In this study there were no pericardial complications with the use of this dedicated TAVI guidewire.
Abbreviations
PTFE: polytetrafluoroethylene
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) has become the standard of care for extreme surgical risk patients with symptomatic severe aortic stenosis, and an alternative to open surgery in those deemed high risk1,2. While the results of TAVI continue to improve with improved operator skill, improving technology and better patient selection, there is still a significant morbidity and mortality associated with the procedure3. One well described complication of TAVI is ventricular perforation and pericardial effusion resulting in pericardial tamponade. This can occur due to left ventricular perforation during any stage of TAVI and usually occurs as a direct result of movements of the stiff wire. Perforation can also occur in the right ventricle from insertion of temporary venous pacing wires. The treatment of both is immediate pericardiocentesis and, in the case of left ventricle perforation, often conversion to open thoracotomy and repair4.
The currently available guidewires used (Amplatz Super Stiff™; Boston Scientific, Natick, MA, USA, and Lunderquist® Extra Stiff; Cook Medical, Bloomington, IN, USA ) were not designed for TAVI procedures and have a rapid transition from a stiff portion (required in order to deliver the valve) to a floppy portion (designed to limit damage to the area in which the wire terminates). These wires share the common feature of a stainless steel core which terminates some distance (e.g., 1 cm, 3 cm or 6 cm) from the tip. There is therefore a discrete transition point at which the wire can kink, and the shoulder so formed can become traumatic. To achieve the optimal shape to sit safely in the ventricle for TAVI, the operator must bend the wire, during which procedure the central core can be damaged or the desired shape may not be achieved. Of 88 TAVI cases performed in our centre prior to the use of the dedicated TAVI wire, one patient sustained a ventricular perforation secondary to a stiff wire and required emergency surgery. The purpose of this study was to design and assess a dedicated guidewire, custom-made to improve safety for TAVI procedures from the transfemoral, subclavian or direct aortic approach.
Methods
DEDICATED TAVI WIRE
The aim of this prospective, first-in-man, open label, non-randomised feasibility study was to evaluate the safety and performance of a specifically designed, dedicated TAVI guidewire. We set out to design a guidewire that would have a very subtle transition point, would be kink-resistant, would have sufficient stiffness to allow delivery of the currently available TAVI systems through tortuous anatomy, and would have a pre-shaped terminal portion which would have shape-holding memory in order to sit comfortably within the left ventricle in a stable atraumatic manner.
We therefore designed a dedicated TAVI guidewire with a central stainless steel core tapering gradually to the tip, with a pre-shaped curve at the end where the curve performs at least one revolution or loop with a decreasing radius. The wire has an extremely subtle/smooth transition phase from stiff to soft that commences at the start of the curvature. We proposed that this pre-shaped curve (Figure 1) would reduce the force to which the left ventricle would be exposed during wire manipulation. The wire has a PTFE coating at the tip.
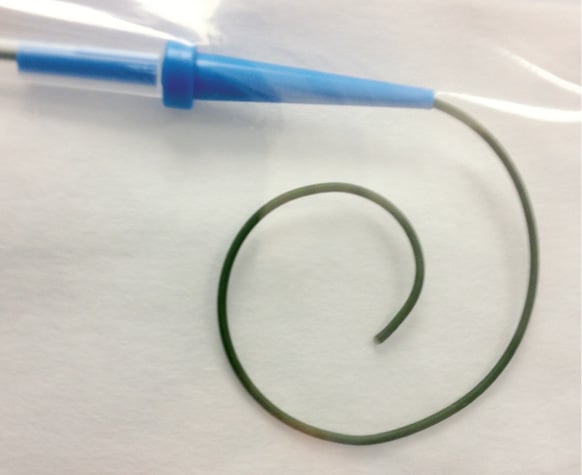
Figure 1. Dedicated TAVI guidewire. Note special tip shape, stainless steel core, PTFE-coated, 0.030”-0.035”
A number of designs and iterations were manufactured in prototype form and bench-tested. Finally, an acceptable design was selected on the basis of shaft stiffness, transition characteristics and terminal shape (EPflex Feinwerktechnik GmbH, Dettingen, Germany) (patent application priority date GB/12.02.09/GBA 0902339). Having designed the guidewire, we carried out a first-in-man, open label, non-randomised feasibility study to evaluate the safety and performance of the guidewire during TAVI procedures with both commercially available valves from a variety of approaches.
PATIENT POPULATION
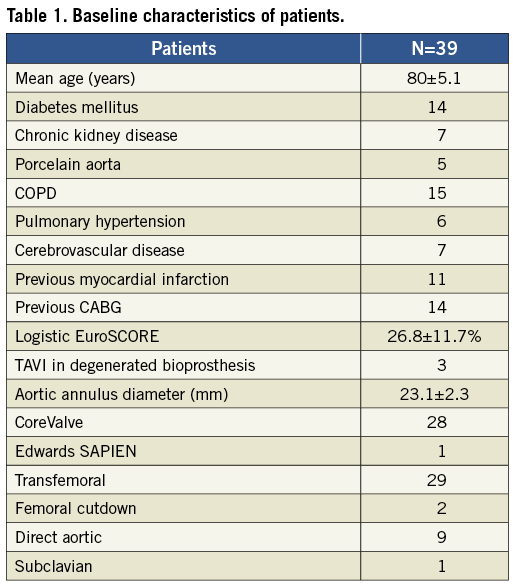
From May 2011 to August 2012 we prospectively enrolled 39 consecutive patients referred for TAVI and used the dedicated TAVI guidewire for their procedure in our institution. Patient screening included detailed clinical examination, transthoracic echocardiography, transoesophageal echocardiography, CT scanning, and coronary and peripheral angiography. All patients with symptomatic severe aortic stenosis who were felt to have a prohibitive surgical risk were assessed by a multidisciplinary heart team comprising cardiothoracic surgeons, interventional cardiologists and stroke neurologists/elderly care physicians. A consensus decision was then reached. Eligibility for TAVI was based on high or prohibitive surgical risk, non-cardiac life expectancy >1 year and anatomy suitable for TAVI. All patients gave written informed consent for the use of the dedicated TAVI wire during the procedure. The study was approved by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and Medical Research Ethics Committee.
TAVI PROCEDURE
All procedures were performed under general anaesthesia with fluoroscopic guidance in a standard cardiac catheterisation laboratory by a dedicated team of experienced operators including an interventional cardiologist, a cardiothoracic surgeon, an echocardiologist and a cardiac anaesthetist. The cardiothoracic surgeon acted as first operator in direct aortic access and subclavian cases and second operator in transfemoral cases. Intraprocedural transoesophageal echocardiography was used for all cases. Both the CoreValve Revalving System (CRS) (Medtronic Inc., Minneapolis, MN, USA) and the Edwards SAPIEN XT™ (Edwards Lifesciences, Irvine, CA, USA) valves were used in performing TAVI procedures. The CoreValve system was used as the default valve while the Edwards valve was used for cases with small annulus sizes. Step-by-step technical details of the transfemoral and direct aortic approaches have been described in detail previously5-7. The direct aortic approach was used as the default access approach of choice for those patients with unsuitable peripheral anatomy for transfemoral TAVI. The aortic valve was crossed using an Amplatz Left 1 (AL1) diagnostic catheter and a conventional straight 0.035” guidewire. The straight guidewire was removed and an exchange length 0.035” guidewire placed within the left ventricle. The AL1 catheter was then exchanged for a 5 Fr pigtail catheter and the aortic valve gradient recorded. The TAVI guidewire was then gradually advanced into the left ventricle while removing the pigtail catheter, thus allowing the TAVI guidewire to adopt its pre-shaped form (Figure 2 and Figure 3). At the end of the procedure the wire was retrieved from the left ventricle using a pigtail catheter to facilitate safe wire removal.
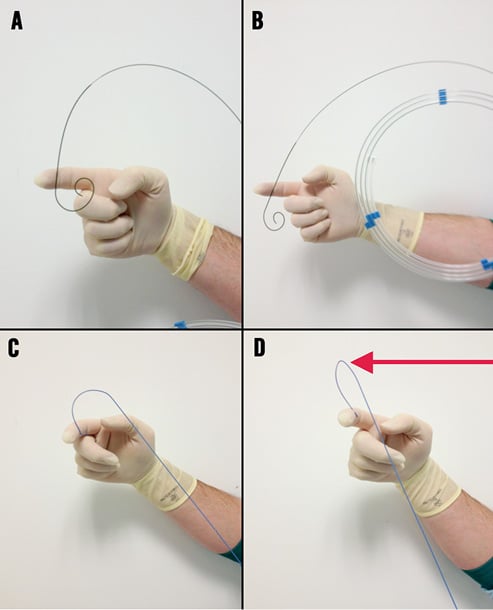
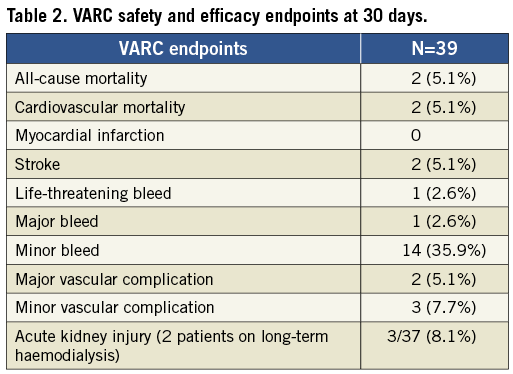
Figure 2. A) and B) show TAVI guidewire showing kink-resistant properties to applied stress. C) and D) show the Amplatz Super Stiff™ wire under minor stress (C) and increased stress (D) with the visible transition point of stiff to floppy wire segment causing a “shoulder” which may be traumatic within the ventricle if the wire is moved forward.
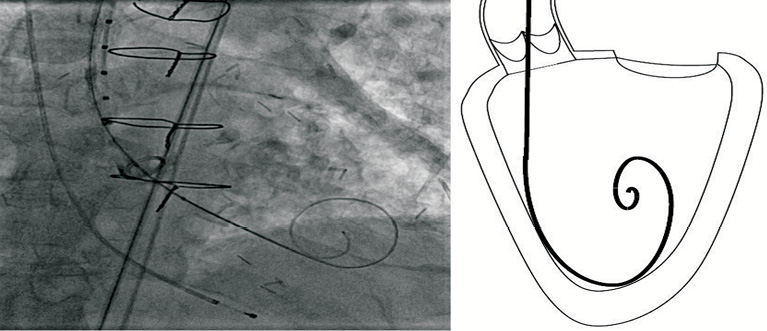
Figure 3. TAVI guidewire during transfemoral case. Note the optimal position in the ventricle and smooth curve which allows wire adjustment without ventricular trauma.
ENDPOINTS
The primary performance endpoint was defined as successful deployment of a transcatheter aortic valve using the guidewire, successful recovery of the delivery system and successful retrieval of the guidewire through a pigtail catheter. The valve had to be deployed without the need to reshape the guidewire, or the need to revert to a different guidewire. The primary safety endpoint was avoidance of device-related complications including death, ventricular perforation with pericardial effusion, and stroke.
Secondary endpoints were the qualitative evaluation of the performance of the wire, including wire displacement from the ventricle, the need for repositioning of the wire, damage to the aortic valve, kinking, difficulty advancing or retrieving the valve delivery system over the wire, and wire damage. Tracking times of the aortic valve delivery system on the wire and valve deployment times were recorded. The data were collected in a comprehensive prospective database which was updated by the operators following each TAVI case using the guidewire.
Results
From May 2011 to August 2012 we prospectively enrolled 39 patients for TAVI with a dedicated TAVI guidewire. The baseline characteristics of the patients are illustrated in Table 1. In our cohort 38 patients were treated with the CoreValve (n=29 transfemoral access, n=8 direct aortic access, and n=1 subclavian access) and one patient with the Edwards SAPIEN valve via a direct aortic access. The dedicated TAVI guidewire was successfully used in all 39 cases.
PRIMARY AND SECONDARY ENDPOINTS
The primary performance endpoint of successful valve deployment and retrieval of the delivery system using the dedicated TAVI guidewire was reached in all 39 cases. With respect to the primary safety endpoint there were no cases of intraprocedural pericardial effusion, tamponade or ventricular perforation and no cases of guidewire-related death or stroke.
With respect to the secondary endpoints there were no cases of wire displacement from the ventricle. One patient with an extremely tortuous and horizontal aorta needed the wire to be repositioned after initial delivery by reinserting the pigtail catheter. A second patient, with tight xenograft stenosis, also needed repositioning of the wire due to unsatisfactory positioning prior to TAVI implantation. There were no cases of aortic valve or aortic wall damage, no wire kinking was seen, there was no difficulty in advancing or retrieving the valve delivery system, and there were no cases of wire damage. Furthermore, we observed that the wire shape was maintained throughout the procedure and the wire could be advanced if necessary towards the apex of the left ventricle safely and atraumatically, maintaining the loop. There were no cases where a second wire was necessary due to damage to the wire. The Valve Academic Research Consortium (VARC) safety and efficacy outcomes at 30 days are reported in Table 28.
One patient developed a pericardial effusion which was related to removal of a temporary pacing wire on day three. This patient developed immediate cardiac tamponade following removal of a right ventricular apical temporary wire and, despite percutaneous drainage and attempted repair of the right ventricular perforation, the patient died. This was unrelated to the use of the guidewire.
A second extreme risk patient with severe left ventricular dysfunction and clinical heart failure developed pulmonary oedema following the procedure. He died on day five from multi-organ failure. This was unrelated to the use of the guidewire.
In one patient the valve was displaced into the ventricle during attempted valve-in-valve treatment for paravalvular aortic regurgitation and this patient proceeded to surgical aortic valve replacement.
The mean tracking time of the valve delivery system over the guidewire was 1.3±0.6 minutes and the mean time of TAVI deployment was 11.1±7.5 minutes.
Discussion
Many thousands of TAVI procedures have been carried out using conventional guidewires, and experienced operators have developed techniques to overcome the potential problems which can arise. However, the currently available guidewires were not designed for delivery of transcatheter valves, or to be placed within the heart. These wires need reshaping of the distal end prior to insertion into the ventricle and there is a significant variation and efficacy to this reshaping process. The design of currently available wires, with the core terminating before the tip, means a transition point can kink causing a shoulder. Extreme aorto-iliac tortuosity, horizontal aorta or small left ventricular cavity are anatomical factors which increase the difficulty of delivering a valve and can lead to more force being transmitted to the left ventricular apex via the guidewire. This increases the risk of ventricular perforation which is a well-recognised life-threatening complication of the procedure, not uncommon during the learning curve. The reported incidence of pericardial tamponade after TAVI varies in the literature from 0-7%3, and most authors do not specify the cause of tamponade. While the introduction of the standardised endpoint definitions by the Valve Academic Research Consortium (VARC) have been a great step forward in TAVI reporting, it has made it more difficult to extract true cases of pericardial effusion or tamponade from some recent literature where tamponade is now included in cardiovascular mortality only9,10. A recent pooled analysis of causes of perioperative mortality after transcatheter valve replacement (12 studies examining 1,223 patients) showed that at one month 10.1% of deaths were due to pericardial tamponade while 39% of “in-lab” mortality was due to cardiac perforation causing pericardial tamponade4.
This study represents the first description of the design and assessment of a dedicated pre-shaped guidewire specifically developed for TAVI. The aims were to design a guidewire which would have a pre-shaped curve to the terminal portion, which could be safely placed within the ventricle, would not perforate the ventricle, even when advanced, and would retain the shape during wire and valve manipulation. Furthermore, the wire is stiffer than conventional wires, thus allowing valve delivery through very tortuous anatomy. Another potential benefit of increased shaft stiffness is the ability to increase the coaxial position of the TAVI system lying obliquely in a horizontal aorta, by pushing on the guidewire when placed within the ventricle.
While experienced operators may have become accustomed to using conventional wires safely, we propose that this wire has some specific properties that make it preferable to the currently available wires used for TAVI, and these may be of particular benefit to inexperienced operators and those on a learning curve. The shape of the loop maintains contact with the ventricular walls and prevents accidental movement - either forwards, potentially causing ventricular perforation, or backwards out through the valve, which can occur when tension is built up on the delivery system during deployment. This is particularly useful in difficult cases where the wire becomes one less thing to focus upon. The loop also allows for repositioning once in the ventricle without the need for reintroducing a pigtail or AL1 catheter. The slight but noticeable increase in stiffness of the shaft, compared to currently available wires, facilitates valve tracking through difficult anatomy and can speed up procedure time. Furthermore, the increased shaft stiffness facilitates manipulation and repositioning of a TAVI delivery system as it lies across the native aortic valve. By pushing on the wire, the TAVI delivery system can be made to lie more horizontally and hence more coaxial in a horizontal aorta, increasing accuracy of valve positioning. The pre-shaped curve means this manoeuvre is safer than with a standard wire and the increased stiffness of the shaft allows more force to be transmitted to the TAVI delivery system.
A particular advantage of the dedicated TAVI guidewire was found to be the ability to move the wire within the ventricle without loss of shape. The size of the loop prevented the wire from being inadvertently pulled out of the ventricle during sheath/delivery system exchange. The increased shaft stiffness of the wire was also shown to be of benefit in extremely tortuous aortic anatomy and when pushing the delivery system through degenerative surgical bioprostheses.
Another advantage is that the constant preformed loop does not require further shaping and gives some consistence to the procedure, as seen in Figure 4. Operators themselves often find that they are not able to make the wire shape exactly the same on each occasion. This means that from time to time they are faced with a wire shape that is suboptimal within the ventricle and a preventable complication can occur. Furthermore, the operator-shaped wires do not always maintain their curve when exiting from the delivery catheter, whereas we did not notice any loss of wire shape when exiting the diagnostic catheter in the left ventricle.
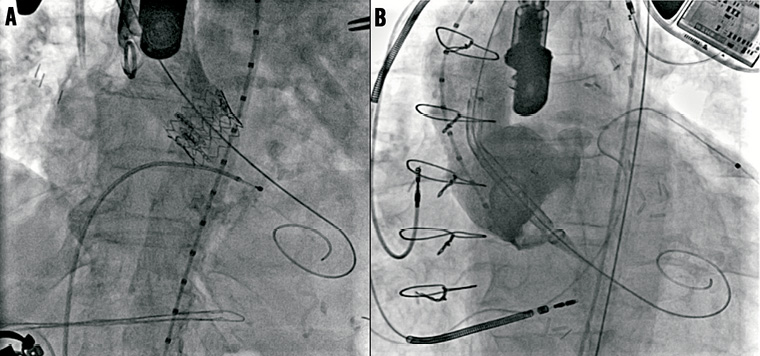
Figure 4. TAVI guidewire during direct aortic access Edwards SAPIEN and transfemoral access CoreValve (valve-in-valve) cases. Note that despite the different orientation the wire curve shape remains constant.
Significant variation exists between operators in shaping and manipulation of the currently available guidewires for TAVI use. Patient factors such as extreme aortic or iliac tortuosity, horizontal aorta and small ventricular cavity all increase the difficulty of TAVI and put more transmitted force onto the wire tip which is in contact with the ventricle, increasing the potential for wire-related complications. In some cases (Figure 5) the desired wire position or shape is not able to be achieved within the ventricle and the operator must exercise extreme caution to avoid perforation.
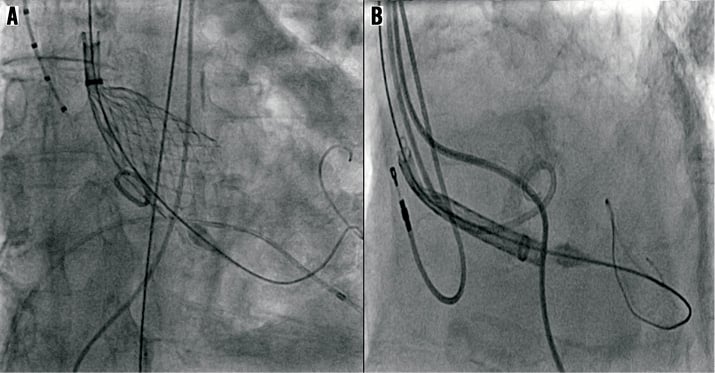
Figure 5. Suboptimal wire shapes and positions for TAVI using conventional wires. Note that the transition point from stiff to soft wire portion is in direct contact with the apex of the left ventricle, increasing the chances of ventricular perforation.
Limitations
This is a small, non-randomised feasibility study of a first-generation dedicated TAVI guidewire and is not large enough to form conclusions about any safety or efficacy advantage over the available guidewires used for TAVI. It does, however, serve as a proof of concept for this technology, but it is possible that a variety of sizes of terminal loop will be desirable. We only assessed one size.
Conclusions
This represents the first description of a dedicated TAVI guidewire to treat patients with a transcatheter aortic valve. The wire is easy to place, safe to adjust within the ventricle and the stiffness of the wire facilitates valve delivery system tracking through tortuous anatomy. In this study there have been no cases of ventricular perforations or other wire-related complications with the use of this dedicated TAVI guidewire.
With the continuing improvement of the currently available valves and their delivery systems as well as operator experience, we have seen continually improving outcomes for patients undergoing TAVI. We should now look for procedural solutions to improve every aspect of the TAVI procedure associated with procedural complications, including the guidewire. It is logical now to use specifically designed, dedicated adjunctive tools for TAVI with the goal of making it a safe and predictable interventional procedure.
Conflict of interest statement
S.J.D. Brecker and J-C. Laborde receive consultancy fees from Medtronic. The other authors have no conflicts of interest to declare.

