Introduction
The SavvyWire (Opsens Medical) is a new 0.035" preshaped guidewire with dedicated pacing properties and a distal pressure sensor allowing for continuous haemodynamic pressure monitoring. This study aimed to determine the safety and efficacy of the SavvyWire during transcatheter aortic valve implantation (TAVI).
Methods
This was a prospective, observational study including patients with severe symptomatic aortic stenosis undergoing TAVI. The study was approved by Health Canada and the local Ethics Committee of each participating centre. All patients provided signed informed consent for participating in the trial (ClinicalTrials.gov: NCT05082337).
SavvyWire
The SavvyWire is a 280 cm long, stiff 0.035" pressure wire (Figure 1A), which is connected to the OptoMonitor (Opsens Medical), a system which displays pressure signals and TAVI-specific metrics (Figure 1B). The guidewire is designed for unipolar pacing with unique conductive and insulation properties. Pacing is achieved by connecting the cathode of an external pacemaker to a pacing connection zone on the shaft, while the anode is connected to a subcutaneous needle in the patient's groin (Figure 1C, Figure 1D).
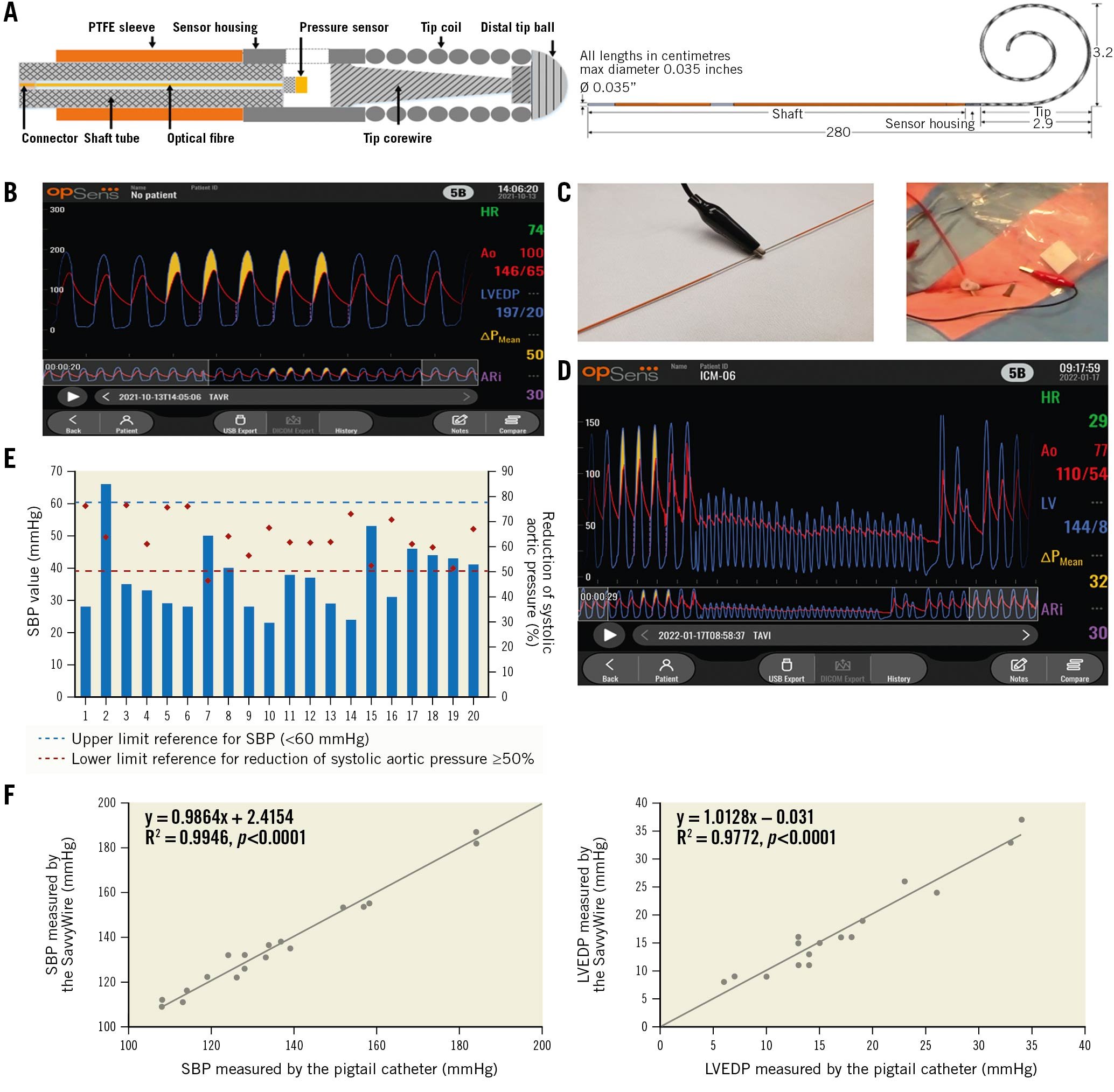
Figure 1. Opsens SavvyWire design and properties. A. SavvyWire high-level design. The shaft section is a stainless-steel tube covered with a PTFE sleeve that provides lubricity for advancing the valve delivery catheter and electrical insulation for rapid pacing. A short section, the sensor housing, joins the shaft to the tip as well as securing the pressure sensor. It also includes a window for the exposure of the pressure sensor to the blood in the left ventricle. The tip has a more flexible distal end and is preshaped into a spiral form for anchoring and to preserve non-traumatic properties during its deployment in the ventricle. The tip comes in 2 standard sizes: extra-small (XS, 3.2 cm) and small (S, 4.2 cm). B. Playback interface of the Opsens OptoMonitor. C. Rapid pacing installation. D. Example of ventricular and systolic aortic pressure reduction under rapid pacing with the SavvyWire. E. Individual data on SBP pressure and reduction of SBP during left ventricular pacing. F. Scatterplot showing the relationship between the SavvyWire and pigtail catheter measurements of SBP and LVEDP. LVEDP: left ventricular end-diastolic pressure; PTFE: polytetrafluoroethylene; SBP: systolic blood pressure
The study procedures and procedural steps are summarised in Supplementary Figure 1.
Study outcomes
The safety primary outcome included the absence of major procedural complications related to the SavvyWire.
The efficacy primary outcomes consisted of (i) effective rapid pacing runs, defined as an adequate ventricular pacing capture, with no capture loss and leading to a reduction of aortic pressure of ≥50% and/or a systolic blood pressure (SBP) of <60 mmHg, and (ii) accurate pressure recordings, defined as pressure wire measurements similar to those obtained simultaneously with a pigtail catheter (difference <5 mmHg).
Statistical analysis
The sample size was empirically estimated at 20 patients. SBP and left ventricular end-diastolic pressure (LVEDP) measurements, obtained simultaneously with the pigtail and the SavvyWire, were plotted using scatter plots and evaluated using the Pearson’s correlation. The means for the SavvyWire and pigtail measurements were compared using paired sample t-tests. Scatter plots and Bland-Altman plots were conducted for the visualisation of differences between the pigtail and the SavvyWire. Statistical significance was established at a p-value <0.05.
Results
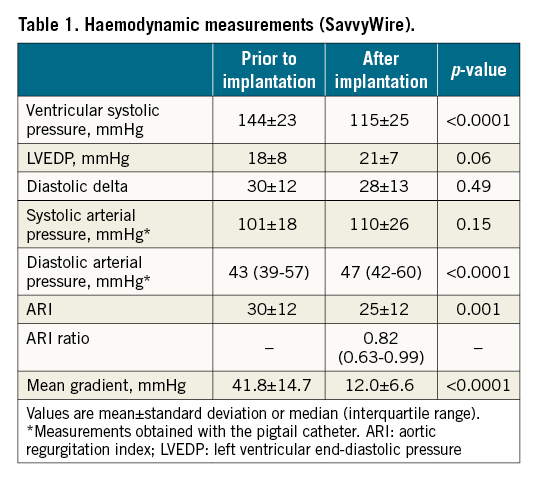
The main clinical characteristics of the study population are shown in Supplementary Table 1. Twelve patients (60%) received an Evolut PRO+ valve (Medtronic) and 8 patients (40%) received a SAPIEN 3 Ultra valve (Edwards Lifesciences). Appropriate left ventricular pacing (180-220 bpm) was achieved in all patients, with a reduction of SBP of at least 50% and/or a systolic pressure value <60 mmHg in all cases (Figure 1E). Procedural haemodynamic measurements are presented in Table 1. There were no cases of pacing capture failure, and the transcatheter heart valve (THV) systems were adequately deployed in all patients. No procedural mortality, stroke, guidewire kink, valve malposition or ventricular perforation were reported (Supplementary Table 2). There were no events related to the SavvyWire. One patient had an episode of complete heart block following valve implantation, and ventricular pacing was ensured by the SavvyWire while the valve delivery system was removed and a temporary pacing lead was inserted in the right ventricle.
Accurate recording of pressure measurements during the TAVI procedure was achieved in all patients. No differences in the SBP and LVEDP measurements obtained with the pigtail and the SavvyWire were found (139.6±26.6 mmHg vs 139.8±26.6 mmHg; p=0.68, and 16.2±9.3 mmHg vs 16.4±9.0 mmHg; p=0.72, for SBP and LVEPD, respectively) (R2=0.99; p<0.0001, and R2=0.98; p˂0.0001, for SBP and LVEDP respectively) (Figure 1F). Bland-Altman plots showed the agreement between the measurements of SBP and LVEPD, with no value out of the reference zone (5 mmHg) and no out-of-control points (mean±1.96 standard deviation [SD]) (Supplementary Figure 2).
Discussion
To date, left ventricular pacing has been performed with non-dedicated pacing wires12. Hensey et al reported the use of a left ventricular guidewire with dedicated pacing properties in TAVI procedures (Wattson wire; Teleflex)3. However, this wire is currently not available.
The current study showed the efficacy of the SavvyWire for adequate rapid pacing runs during TAVI procedures with balloon-expandable and self-expanding valves, with no cases of pacing capture failure. Also, if backup pacing is required post-THV deployment, the wire length (280 cm), along with its electrical insulation properties, allows for continuous pacing while the THV delivery system is removed from the sheath and a temporary pacing lead is inserted in the right ventricle.
Several studies have shown the importance of evaluating valve haemodynamics by catheterisation measurements (vs Doppler echocardiography) during TAVI45.
However, this requires multiple catheter-wire exchanges, which may potentially increase the risk of complications. Using a support guidewire with pressure measurement capabilities facilitated the haemodynamic evaluation of transcatheter valve performance in a safer and more rapid manner.
Limitations
The study had a small sample size. Further studies are warranted.
Conclusions
The results of this study showed the safety and efficacy of the SavvyWire for TAVI. The use of this wire would simplify the TAVI procedure (no right ventricular pacing, no catheter-wire exchanges for haemodynamic measurements) and facilitate the clinical decision-making process.
Acknowledgments
Dr. Rodés-Cabau holds the Research Chair “Fondation Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions.
Funding
This study was supported by Opsens Medical, Quebec City, Canada.
Conflict of interest statement
J. Rodés-Cabau is a consultant for and has received institutional research grants from Opsens Medical; has received grants from Edwards Lifesciences, and Medtronic; consulting fees from Medtronic, and Opens; and speaker fees/honoraria from Edwards Lifesciences, and Medtronic. R. Ibrahim is a consultant for and has received speaker fees from Opsens Medical. M. Picard-Deland and S. Lalancette are employees of Opsens Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

