Abstract
The introduction of the so-called newer-generation transcatheter aortic valve implantation (TAVI) devices has led to a dramatic reduction in the incidence of complications associated with the procedure. However, preliminary data suggest that conduction abnormalities (particularly new-onset atrioventricular block and left bundle branch block) remain a frequent complication post TAVI. Although inconsistencies across studies are apparent, new-onset conduction abnormalities post TAVI may be associated with higher incidences of mortality, sudden cardiac death and left ventricular dysfunction. Strategies intended both to reduce the risk and to improve the management of such complications are clearly warranted. In fact, the indication and timing of permanent pacemaker implantation are frequently individualised according to centre and/or operator preference. Currently, studies assessing the impact of these complications and the optimal indications for permanent cardiac pacing are underway. In this article, we review the data available on the incidence and impact of conduction disturbances following TAVI, and propose a strategy for the management of such complications.
Introduction
The aortic valve has close spatial proximity to the conduction system, and in particular to the bundle of His and left bundle branch1,2. As a result of this anatomical interaction, conduction abnormalities are frequently observed in patients with calcific aortic stenosis, with increasing rates observed following aortic valve interventions, in particular transcatheter aortic valve implantation (TAVI). The stent frame of the valve prosthesis, in addition to the delivery system and stiff guidewires used during the procedure, may exert mechanical stress on the ventricular wall bounding the aortic valve, including the ventricular septum and conduction system. The location, magnitude and duration of these mechanical forces and patients’ anatomical and pathological conditions may determine the type (mostly atrioventricular [AV] block or left bundle branch block [LBBB]) and duration of these conduction abnormalities (transient or persistent).
Incidence and predictors of conduction disturbances post TAVI
Overall, the rate of new-onset LBBB post TAVI is ~27% (ranging from 4-57%)1 and the rate of permanent pacemaker (PPM) implantation ~17% (from 2-51%)3. Wide variations have been reported across studies and according to valve type (Table 1). In general terms, the incidence of both new-onset LBBB and PPM implantation is higher following use of the self-expanding CoreValve (Medtronic Inc., Minneapolis, MN, USA) system (~48% and 28%, respectively) compared with the balloon-expandable Edwards SAPIEN/SAPIEN XT (Edwards Lifesciences LLC, Irvine, CA, USA) valve (~14% and 6%, respectively)1-3. Indeed, the increased risk of PPM requirement associated with the CoreValve prosthesis compared with the Edwards SAPIEN/SAPIEN XT valve has been confirmed in a randomised trial (37.6% vs. 17.3%, p<0.001)4. A slow but significant reduction in the rate of conduction abnormalities and PPM requirement associated with both transcatheter valve types has been observed over time1,5. This may be related to improvements in delivery systems, increased experience and knowledge of the factors associated with conduction disturbances post TAVI, in addition to the implementation of more restrictive indications for PPM implantation5.
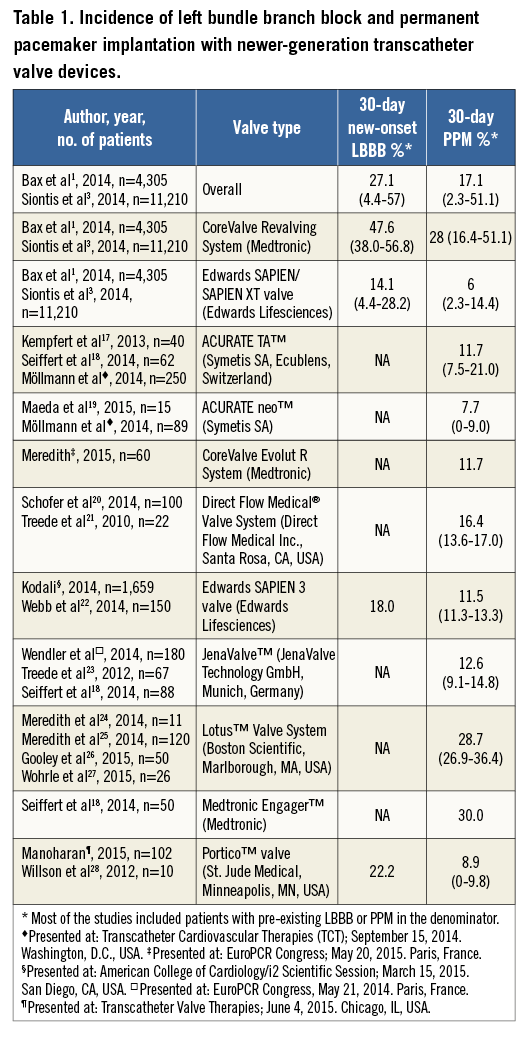
In recent times, newer iterations of transcatheter valve designs intended to improve the results of TAVI have been introduced. Preliminary results associated with these new devices have shown a dramatic decrease in the incidence of some major periprocedural complications, without impacting beneficially on the incidence of conduction abnormalities. Data on the risk of new-onset LBBB associated with these devices are scarce (Table 1). However, these early results show no reduction in the rate of PPM implantation associated with most newer transcatheter aortic valves (~13%, range from 8-30%) (Table 1), suggesting that the retrievability/repositioning capabilities of most of these newer prostheses fail to reduce the occurrence of conduction abnormalities post TAVI. Furthermore, no additional features have been specifically developed to reduce the risk of these complications. No significant decrease in the rate of new conduction disturbances post TAVI is therefore anticipated in the near future.
Both patient clinical characteristics and procedural features have been reported as predictors of new-onset LBBB and PPM requirement. Clinical predictors associated with an increased risk of new-onset LBBB include the presence of pre-existing conduction abnormalities (longer baseline QRS duration) and TAVI within the native aortic valve (as opposed to valve-in-valve procedures)1,2. Valve prosthesis type (self-expandable) and the depth of implantation are the only modifiable procedural factors predicting the occurrence of new-onset LBBB1. Likewise, clinical factors such as male gender, absence of prior valve surgery, the presence of porcelain aorta and pre-existing conduction abnormalities (mainly pre-existing right bundle branch block, but also pre-existing left anterior hemiblock and first degree AV block) are independent predictors of the need for PPM post TAVI1. Similarly, intraprocedural AV block and modifiable factors such as implantation depth, use of the CoreValve system, and balloon predilatation are each independently associated with an increased likelihood of PPM implantation1-3. Nonetheless, up to now only a more “aortic” prosthesis position (≤6 mm below the aortic annulus) and use of the Edwards SAPIEN/SAPIEN XT valves (compared with the CoreValve system) have been shown to reduce the need for PPM implantation after TAVI5.
Management of conduction disturbances post TAVI
It is apparent that strict adherence to current PPM guidelines post TAVI reduces the frequency of this complication5. The main reason for PPM implantation post TAVI relates to the occurrence of complete or high degree AV block3, with about 33% and 50% of PPMs being implanted within the first 24 and 48 hours post TAVI, respectively6,7. This contrasts with current European recommendations suggesting a period of clinical observation and ECG monitoring for up to seven days prior to implanting a PPM in patients with high degree or complete AV block in order to determine whether rhythm disturbances post TAVI are temporary or permanent (Class I, Level of Evidence C)8. This observation period may be shortened only in cases of complete AV block with slow escape rhythm8. Such a strategy of more prolonged ECG monitoring post TAVI prior to PPM implantation is supported by the results of studies showing that (i) a significant proportion of conduction abnormalities resolves early within the post-TAVI period, and (ii) there is increased risk of late mortality or repeat hospitalisations for heart failure associated with cardiac pacing, particularly in patients with low LVEF and higher rates of PPM dependency2. However, incorporation of these strategies into protocols for earlier hospital discharge following TAVI (and increased cost-effectiveness) will be challenging.
Although early TAVI studies failed to demonstrate an association between PPM implantation and mortality or MACE over a mean follow-up of ~3 years, recent results have suggested a negative impact of PPM implantation on the evolution of left ventricular ejection fraction post TAVI6,9. Also, PPM implantation post TAVI may lack clinical benefit in a significant proportion of patients due to recovery of AV conduction during the follow-up period1. Nonetheless, the risk/benefit and cost/benefit ratio of continuous ECG monitoring (often with associated temporary pacing) for a period of seven days post TAVI to allow possible rhythm recovery before implantation of a PPM requires confirmation in future studies. In fact, this strategy competes with current trends towards reducing the length of hospital stay post TAVI in order to limit costs and complications. Interestingly, the adoption of early discharge strategies (within 24-72 hours) post TAVI has not been associated with an increased risk of re-hospitalisation or sudden cardiac death10,11, suggesting that 24 hours of ECG monitoring (instead of 72 hours as recommended in ESC guidelines) may be sufficient in patients with no conduction abnormalities immediately following the procedure.
Interestingly, conduction abnormalities post TAVI often seem to be present pre-procedure and remain undetected until post-procedural ECG monitoring is performed. Therefore, ECG monitoring for at least 24 hours pre TAVI could allow prompt identification and treatment of conduction abnormalities which are not expected to resolve and could lead to an overall reduction in length of hospital stay12.
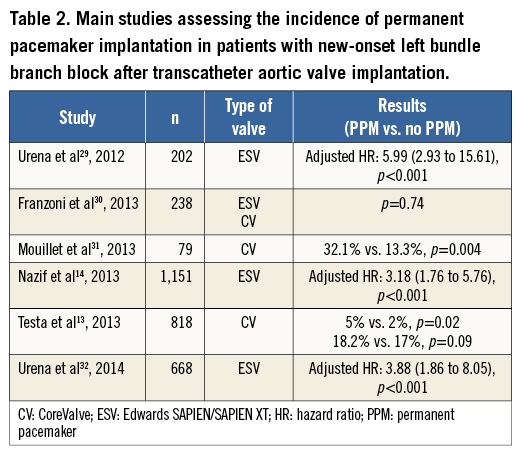
In addition to complete or high degree AV block, sick sinus syndrome or severe bradycardia and the occurrence of new-onset persistent LBBB are other reasons to consider PPM implantation post TAVI3. No evidence exists on a causal relationship between TAVI and the occurrence of sinus node disease or severe bradycardia due to causes other than AV block, and there are no current specific indications for PPM (other than general recommendations for PPM implantation) in these patients. However, the indications for PPM implantation in patients with new LBBB post TAVI are more controversial. Several studies have shown an increased risk (>3-fold) of late advanced AV block and need for PPM implantation in patients with new-onset persistent LBBB post TAVI (Table 2). In addition, although results have been discordant across studies, the occurrence of new-onset persistent LBBB has been associated with an increased risk of overall mortality and sudden cardiac death, particularly in patients with prolonged QRS duration (>160 msec)13-16. The ongoing MARE (Ambulatory Electrocardiographic Monitoring for the Detection of High-Degree Atrio-Ventricular Block in Patients With New-onset PeRsistent LEft Bundle Branch Block After Transcatheter Aortic Valve Implantation) study (NCT02153307), and the recently commenced “Assessment of the Prognosis of Persistent Left Bundle Branch Block (LBBB) After Transcatheter Aortic Valve Implantation (TAVI ) by an Electrophysiological and Remote Monitoring Risk-adapted Algorithm” study (NCT02482844) will provide insight into the complications associated with this conduction abnormality as well as its optimal therapy. Meanwhile, current indications for PPM implantation appear to be reasonable in certain patients with new persistent LBBB (i.e., those with QRS >160 msec) post TAVI.
Conclusions
The occurrence of new conduction disturbances and of the need for PPM post TAVI remains a major concern due to its high frequency (which is unlikely to decrease in the near future) and potentially negative impact on mid- and long-term outcomes. Use of balloon-expandable valve systems and a high (more aortic) implantation site has been associated with significant reductions of such complications. Limiting the indications for PPM to those strictly recommended in guidelines, with more prolonged periods of ECG monitoring prior to PPM implantation, may ultimately reduce implantation rates. The group of patients with new-onset LBBB post TAVI is particularly challenging, and indications for PPM implantation in such patients remain controversial. Ongoing studies will provide further insights into the risks associated with this complication and its optimal therapy.
Conflict of interest statement
J. Rodés-Cabau has received research grants from Edwards Lifesciences, Medtronic, and St. Jude Medical. M. Urena has no conflicts of interest to declare.

